Chronic immune response dysregulation in MDS pathogenesis
- PMID: 30104218
- PMCID: PMC6182269
- DOI: 10.1182/blood-2018-03-784116
Chronic immune response dysregulation in MDS pathogenesis
Abstract
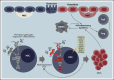
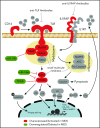
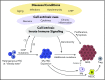
Chronic innate immune signaling in hematopoietic cells is widely described in myelodysplastic syndromes (MDS), and innate immune pathway activation, predominantly via pattern recognition receptors, increases the risk of developing MDS. An inflammatory component to MDS has been reported for many years, but only recently has evidence supported a more direct role of chronic innate immune signaling and associated inflammatory pathways in the pathogenesis of MDS. Here we review recent findings and discuss relevant questions related to chronic immune response dysregulation in MDS.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures
References
-
- Hofmann WK, de Vos S, Komor M, Hoelzer D, Wachsman W, Koeffler P. Characterization of gene expression of CD34+ cells from normal and myelodysplastic bone marrow. Blood. 2002;100(10):3553-3560. - PubMed
-
- Pellagatti A, Cazzola M, Giagounidis A, et al. Deregulated gene expression pathways in myelodysplastic syndrome hematopoietic stem cells. Leukemia. 2010;24:756-764. - PubMed
-
- Maratheftis CI, Andreakos E, Moutsopoulos HM, Voulgarelis M. Toll-like receptor-4 is up-regulated in hematopoietic progenitor cells and contributes to increased apoptosis in myelodysplastic syndromes. Clin Cancer Res. 2007;13(4):1154-1160. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

