CD19 Alterations Emerging after CD19-Directed Immunotherapy Cause Retention of the Misfolded Protein in the Endoplasmic Reticulum
- PMID: 30104252
- PMCID: PMC6189457
- DOI: 10.1128/MCB.00383-18
CD19 Alterations Emerging after CD19-Directed Immunotherapy Cause Retention of the Misfolded Protein in the Endoplasmic Reticulum
Erratum in
-
Correction for Bagashev et al., "CD19 Alterations Emerging after CD19-Directed Immunotherapy Cause Retention of the Misfolded Protein in the Endoplasmic Reticulum".Mol Cell Biol. 2022 Sep 15;42(9):e0032822. doi: 10.1128/mcb.00328-22. Epub 2022 Aug 17. Mol Cell Biol. 2022. PMID: 35976030 Free PMC article. No abstract available.
Abstract
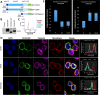
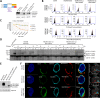
We previously described a mechanism of acquired resistance of B-cell acute lymphoblastic leukemia to CD19-directed chimeric antigen receptor T-cell (CART) immunotherapy. It was based on in-frame insertions in or skipping of CD19 exon 2. To distinguish between epitope loss and defects in surface localization, we used retroviral transduction and genome editing to generate cell lines expressing CD19 exon 2 variants (CD19ex2vs) bearing vesicular stomatitis virus G protein (VSVg) tags. These lines were negative by live-cell flow cytometry with an anti-VSVg antibody and resistant to killing by VSVg-directed antibody-drug conjugates (ADCs), suggestive of a defect in surface localization. Indeed, pulse-chase and α-mannosidase inhibitor assays showed that all CD19ex2vs acquired endoplasmic reticulum (ER)-specific high-mannose-type sugars but not complex-type glycans synthesized in the Golgi apparatus. When fused with green fluorescent protein (GFP), CD19ex2vs (including a mutant lacking the relevant disulfide bond) showed colocalization with ER markers, implying protein misfolding. Mass spectrometric profiling of CD19-interacting proteins demonstrated that CD19ex2vs fail to bind to the key tetraspanin CD81 and instead interact with ER-resident chaperones, such as calnexin, and ER transporters involved in antigen presentation. Thus, even the intact domains of CD19ex2vs cannot be easily targeted with ADCs or current CD19 CARTs but could serve as sources of peptides for major histocompatibility complex (MHC)-restricted presentation and T-cell receptor (TCR)-mediated killing.
Keywords: RNA splicing; immunotherapy; membrane proteins; membrane transport; protein folding.
Copyright © 2018 Bagashev et al.
Figures






References
-
- Topp MS, Gokbuget N, Zugmaier G, Klappers P, Stelljes M, Neumann S, Viardot A, Marks R, Diedrich H, Faul C, Reichle A, Horst HA, Bruggemann M, Wessiepe D, Holland C, Alekar S, Mergen N, Einsele H, Hoelzer D, Bargou RC. 2014. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol 32:4134–4140. doi: 10.1200/JCO.2014.56.3247. - DOI - PubMed
-
- Topp MS, Kufer P, Gokbuget N, Goebeler M, Klinger M, Neumann S, Horst HA, Raff T, Viardot A, Schmid M, Stelljes M, Schaich M, Degenhard E, Kohne-Volland R, Bruggemann M, Ottmann O, Pfeifer H, Burmeister T, Nagorsen D, Schmidt M, Lutterbuese R, Reinhardt C, Baeuerle PA, Kneba M, Einsele E, Riethmuller G, Hoelzer D, Zugmaier G, Bargou RC. 2011. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol 29:2493–2498. doi: 10.1200/JCO.2010.32.7270. - DOI - PubMed
-
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA. 2014. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371:1507–1517. doi: 10.1056/NEJMoa1407222. - DOI - PMC - PubMed
-
- Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, Wolters P, Martin S, Delbrook C, Yates B, Shalabi H, Fountaine TJ, Shern JF, Majzner RG, Stroncek DF, Sabatino M, Feng Y, Dimitrov DS, Zhang L, Nguyen S, Qin H, Dropulic B, Lee DW, Mackall CL. 2018. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med 24:20–28. doi: 10.1038/nm.4441. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
