Nitazoxanide Inhibits Human Norovirus Replication and Synergizes with Ribavirin by Activation of Cellular Antiviral Response
- PMID: 30104275
- PMCID: PMC6201076
- DOI: 10.1128/AAC.00707-18
Nitazoxanide Inhibits Human Norovirus Replication and Synergizes with Ribavirin by Activation of Cellular Antiviral Response
Abstract
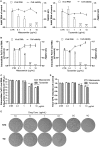
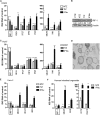
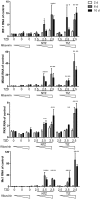
Norovirus is the main cause of viral gastroenteritis worldwide. Although norovirus gastroenteritis is self-limiting in immunocompetent individuals, chronic infections with debilitating and life-threatening complications occur in immunocompromised patients. Nitazoxanide (NTZ) has been used empirically in the clinic and has demonstrated effectiveness against norovirus gastroenteritis. In this study, we aimed at uncovering the antiviral potential and mechanisms of action of NTZ and its active metabolite, tizoxanide (TIZ), using a human norovirus (HuNV) replicon. NTZ and TIZ, collectively referred to as thiazolides (TZD), potently inhibited replication of HuNV and a norovirus surrogate, feline calicivirus. Mechanistic studies revealed that TZD activated cellular antiviral response and stimulated the expression of a subset of interferon-stimulated genes (ISGs), particularly interferon regulatory factor 1 (IRF-1), not only in a Huh7 cell-based HuNV replicon, but also in naive Huh7 and Caco-2 cells and novel human intestinal organoids. Overexpression of exogenous IRF-1 inhibited HuNV replication, whereas knockdown of IRF-1 largely attenuated the antiviral activity of TZD, suggesting that IRF-1 mediated TZD inhibition of HuNV. By using a Janus kinase (JAK) inhibitor, CP-690550, and a STAT1 knockout approach, we found that TZD induced antiviral response independently of the classical JAK-signal transducers and activators of transcription (JAK-STAT) pathway. Furthermore, TZD and ribavirin synergized to inhibit HuNV replication and completely depleted the replicons from host cells after long-term treatment. In summary, our results demonstrated that TZD combated HuNV replication through activation of cellular antiviral response, in particular by inducing a prominent antiviral effector, IRF-1. NTZ monotherapy or combination with ribavirin represent promising options for treating norovirus gastroenteritis, especially in immunocompromised patients.
Keywords: IRF-1; cell culture model; nitazoxanide; noroviruses; ribavirin; synergy; tizoxanide.
Copyright © 2018 American Society for Microbiology.
Figures





Similar articles
-
2'-Fluoro-2'-deoxycytidine inhibits murine norovirus replication and synergizes MPA, ribavirin and T705.Arch Virol. 2020 Nov;165(11):2605-2613. doi: 10.1007/s00705-020-04759-4. Epub 2020 Aug 8. Arch Virol. 2020. PMID: 32770483 Free PMC article.
-
Inhibition of Calcineurin or IMP Dehydrogenase Exerts Moderate to Potent Antiviral Activity against Norovirus Replication.Antimicrob Agents Chemother. 2017 Oct 24;61(11):e01095-17. doi: 10.1128/AAC.01095-17. Print 2017 Nov. Antimicrob Agents Chemother. 2017. PMID: 28807916 Free PMC article.
-
IRF-1, RIG-I and MDA5 display potent antiviral activities against norovirus coordinately induced by different types of interferons.Antiviral Res. 2018 Jul;155:48-59. doi: 10.1016/j.antiviral.2018.05.004. Epub 2018 May 10. Antiviral Res. 2018. PMID: 29753657
-
Norovirus: targets and tools in antiviral drug discovery.Biochem Pharmacol. 2014 Sep 1;91(1):1-11. doi: 10.1016/j.bcp.2014.05.021. Epub 2014 Jun 2. Biochem Pharmacol. 2014. PMID: 24893351 Free PMC article. Review.
-
Human norovirus cultivation systems and their use in antiviral research.J Virol. 2024 Apr 16;98(4):e0166323. doi: 10.1128/jvi.01663-23. Epub 2024 Mar 12. J Virol. 2024. PMID: 38470106 Free PMC article. Review.
Cited by
-
Experimental Pharmacotherapy for COVID-19: The Latest Advances.J Exp Pharmacol. 2021 Jan 7;13:1-13. doi: 10.2147/JEP.S255209. eCollection 2021. J Exp Pharmacol. 2021. PMID: 33442304 Free PMC article. Review.
-
The Effect of Nitazoxanide on the Clinical Outcomes in Patients with COVID-19: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.Clin Drug Investig. 2022 Dec;42(12):1031-1047. doi: 10.1007/s40261-022-01213-y. Epub 2022 Oct 31. Clin Drug Investig. 2022. PMID: 36315350 Free PMC article.
-
An Open Label, Adaptive, Phase 1 Trial of High-Dose Oral Nitazoxanide in Healthy Volunteers: An Antiviral Candidate for SARS-CoV-2.Clin Pharmacol Ther. 2022 Mar;111(3):585-594. doi: 10.1002/cpt.2463. Epub 2021 Nov 13. Clin Pharmacol Ther. 2022. PMID: 34699618 Free PMC article. Clinical Trial.
-
2'-Fluoro-2'-deoxycytidine inhibits murine norovirus replication and synergizes MPA, ribavirin and T705.Arch Virol. 2020 Nov;165(11):2605-2613. doi: 10.1007/s00705-020-04759-4. Epub 2020 Aug 8. Arch Virol. 2020. PMID: 32770483 Free PMC article.
-
Human Norovirus Efficiently Replicates in Differentiated 3D-Human Intestinal Enteroids.J Virol. 2022 Nov 23;96(22):e0085522. doi: 10.1128/jvi.00855-22. Epub 2022 Nov 7. J Virol. 2022. PMID: 36342297 Free PMC article.
References
-
- Schwartz S, Vergoulidou M, Schreier E, Loddenkemper C, Reinwald M, Schmidt-Hieber M, Flegel WA, Thiel E, Schneider T. 2011. Norovirus gastroenteritis causes severe and lethal complications after chemotherapy and hematopoietic stem cell transplantation. Blood 117:5850–5856. doi:10.1182/blood-2010-12-325886. - DOI - PMC - PubMed
-
- Morris J, Morris C. 2015. Nitazoxanide is effective therapy for norovirus gastroenteritis after chemotherapy and hematopoietic stem cell transplantation (HSCT). Biol Blood Marrow Transplant 21:S255–S256.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

