α-Neurexins Together with α2δ-1 Auxiliary Subunits Regulate Ca2+ Influx through Cav2.1 Channels
- PMID: 30104341
- PMCID: PMC6596161
- DOI: 10.1523/JNEUROSCI.0511-18.2018
α-Neurexins Together with α2δ-1 Auxiliary Subunits Regulate Ca2+ Influx through Cav2.1 Channels
Abstract
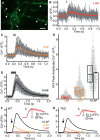
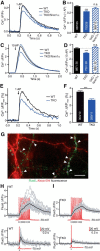
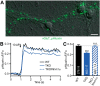
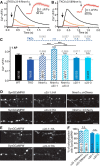
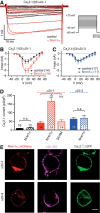
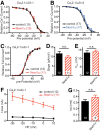
Action potential-evoked neurotransmitter release is impaired in knock-out neurons lacking synaptic cell-adhesion molecules α-neurexins (αNrxns), the extracellularly longer variants of the three vertebrate Nrxn genes. Ca2+ influx through presynaptic high-voltage gated calcium channels like the ubiquitous P/Q-type (CaV2.1) triggers release of fusion-ready vesicles at many boutons. α2δ Auxiliary subunits regulate trafficking and kinetic properties of CaV2.1 pore-forming subunits but it has remained unclear if this involves αNrxns. Using live cell imaging with Ca2+ indicators, we report here that the total presynaptic Ca2+ influx in primary hippocampal neurons of αNrxn triple knock-out mice of both sexes is reduced and involved lower CaV2.1-mediated transients. This defect is accompanied by lower vesicle release, reduced synaptic abundance of CaV2.1 pore-forming subunits, and elevated surface mobility of α2δ-1 on axons. Overexpression of Nrxn1α in αNrxn triple knock-out neurons is sufficient to restore normal presynaptic Ca2+ influx and synaptic vesicle release. Moreover, coexpression of Nrxn1α together with α2δ-1 subunits facilitates Ca2+ influx further but causes little augmentation together with a different subunit, α2δ-3, suggesting remarkable specificity. Expression of defined recombinant CaV2.1 channels in heterologous cells validates and extends the findings from neurons. Whole-cell patch-clamp recordings show that Nrxn1α in combination with α2δ-1, but not with α2δ-3, facilitates Ca2+ currents of recombinant CaV2.1 without altering channel kinetics. These results suggest that presynaptic Nrxn1α acts as a positive regulator of Ca2+ influx through CaV2.1 channels containing α2δ-1 subunits. We propose that this regulation represents an important way for neurons to adjust synaptic strength.SIGNIFICANCE STATEMENT Synaptic transmission between neurons depends on the fusion of neurotransmitter-filled vesicles with the presynaptic membrane, which subsequently activates postsynaptic receptors. Influx of calcium ions into the presynaptic terminal is the key step to trigger vesicle release and involves different subtypes of voltage-gated calcium channels. We study the regulation of calcium channels by neurexins, a family of synaptic cell-adhesion molecules that are essential for many synapse properties. Using optical measurements of calcium influx in cultured neurons and electrophysiological recordings of calcium currents from recombinant channels, we show that a major neurexin variant facilitates calcium influx through P/Q-type channels by interacting with their α2δ-1 auxiliary subunits. These results propose a novel way how neurons can modulate the strength of distinct synapses.
Keywords: calcium channels; cell adhesion molecules; live cell imaging; neurexins; patch-clamp electrophysiology; synaptic transmission.
Copyright © 2018 the authors 0270-6474/18/388277-18$15.00/0.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
