Tyrosyl-tRNA synthetase stimulates thrombopoietin-independent hematopoiesis accelerating recovery from thrombocytopenia
- PMID: 30104364
- PMCID: PMC6126720
- DOI: 10.1073/pnas.1807000115
Tyrosyl-tRNA synthetase stimulates thrombopoietin-independent hematopoiesis accelerating recovery from thrombocytopenia
Abstract
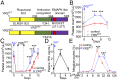
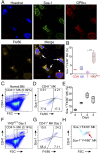
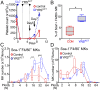
New mechanisms behind blood cell formation continue to be uncovered, with therapeutic approaches for hematological diseases being of great interest. Here we report an enzyme in protein synthesis, known for cell-based activities beyond translation, is a factor inducing megakaryocyte-biased hematopoiesis, most likely under stress conditions. We show an activated form of tyrosyl-tRNA synthetase (YRSACT), prepared either by rationally designed mutagenesis or alternative splicing, induces expansion of a previously unrecognized high-ploidy Sca-1+ megakaryocyte population capable of accelerating platelet replenishment after depletion. Moreover, YRSACT targets monocytic cells to induce secretion of transacting cytokines that enhance megakaryocyte expansion stimulating the Toll-like receptor/MyD88 pathway. Platelet replenishment by YRSACT is independent of thrombopoietin (TPO), as evidenced by expansion of the megakaryocytes from induced pluripotent stem cell-derived hematopoietic stem cells from a patient deficient in TPO signaling. We suggest megakaryocyte-biased hematopoiesis induced by YRSACT offers new approaches for treating thrombocytopenia, boosting yields from cell-culture production of platelet concentrates for transfusion, and bridging therapy for hematopoietic stem cell transplantation.
Keywords: megakaryopoiesis; thrombocytopenia; tyrosyl-tRNA synthetase.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
Conflict of interest statement: R.B., M.-H.D., X.-L.Y., and P.S. have a financial interest in aTyr Pharma, although none specifically for this work.
Figures





Comment in
-
Tyrosyl-tRNA synthetase drives megakaryopoiesis independently of thrombopoietin signaling.J Thromb Haemost. 2019 Apr;17(4):564-566. doi: 10.1111/jth.14361. Epub 2019 Jan 11. J Thromb Haemost. 2019. PMID: 30632668 No abstract available.
Similar articles
-
Inflammatory platelet production stimulated by tyrosyl-tRNA synthetase mimicking viral infection.Proc Natl Acad Sci U S A. 2022 Nov 29;119(48):e2212659119. doi: 10.1073/pnas.2212659119. Epub 2022 Nov 21. Proc Natl Acad Sci U S A. 2022. PMID: 36409883 Free PMC article.
-
Miniaturized 3D bone marrow tissue model to assess response to Thrombopoietin-receptor agonists in patients.Elife. 2021 Jun 1;10:e58775. doi: 10.7554/eLife.58775. Elife. 2021. PMID: 34059198 Free PMC article.
-
Congenital amegakaryocytic thrombocytopenia iPS cells exhibit defective MPL-mediated signaling.J Clin Invest. 2013 Sep;123(9):3802-14. doi: 10.1172/JCI64721. Epub 2013 Aug 1. J Clin Invest. 2013. PMID: 23908116 Free PMC article.
-
[Congenital amegakaryocytic thrombocytopenia (CAMT) - a defect of the thrombopoietin receptor c-Mpl].Klin Padiatr. 2001 Jul-Aug;213(4):155-61. doi: 10.1055/s-2001-16846. Klin Padiatr. 2001. PMID: 11528548 Review. German.
-
Thrombopoietin: biology and clinical potentials.Int J Hematol. 1999 Dec;70(4):216-25. Int J Hematol. 1999. PMID: 10643146 Review.
Cited by
-
Extracellular tyrosyl-tRNA synthetase cleaved by plasma proteinases and stored in platelet α-granules: Potential role in monocyte activation.Res Pract Thromb Haemost. 2020 Sep 14;4(7):1167-1177. doi: 10.1002/rth2.12429. eCollection 2020 Oct. Res Pract Thromb Haemost. 2020. PMID: 33134783 Free PMC article.
-
Thrombocytopenia in dengue infection: mechanisms and a potential application.Expert Rev Mol Med. 2024 Oct 14;26:e26. doi: 10.1017/erm.2024.18. Expert Rev Mol Med. 2024. PMID: 39397710 Free PMC article. Review.
-
The Application of Ethnomedicine in Modulating Megakaryocyte Differentiation and Platelet Counts.Int J Mol Sci. 2023 Feb 5;24(4):3168. doi: 10.3390/ijms24043168. Int J Mol Sci. 2023. PMID: 36834579 Free PMC article. Review.
-
Targeting a thrombopoietin-independent strategy in the discovery of a novel inducer of megakaryocytopoiesis, DMAG, for the treatment of thrombocytopenia.Haematologica. 2023 May 1;108(5):1394-1411. doi: 10.3324/haematol.2022.282209. Haematologica. 2023. PMID: 36546424 Free PMC article.
-
The role of RNA epigenetic modification in normal and malignant hematopoiesis.Curr Stem Cell Rep. 2020 Dec;6(4):144-155. doi: 10.1007/s40778-020-00178-y. Epub 2020 Aug 12. Curr Stem Cell Rep. 2020. PMID: 33777659 Free PMC article.
References
-
- Kim S, You S, Hwang D. Aminoacyl-tRNA synthetases and tumorigenesis: More than housekeeping. Nat Rev Cancer. 2011;11:708–718. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

