N6-methyladenosine modification of hepatitis B virus RNA differentially regulates the viral life cycle
- PMID: 30104368
- PMCID: PMC6126736
- DOI: 10.1073/pnas.1808319115
N6-methyladenosine modification of hepatitis B virus RNA differentially regulates the viral life cycle
Abstract
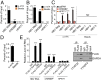
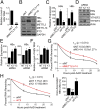
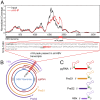
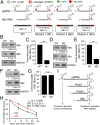
N6-methyladenosine (m6A) RNA methylation is the most abundant epitranscriptomic modification of eukaryotic messenger RNAs (mRNAs). Previous reports have found m6A on both cellular and viral transcripts and defined its role in regulating numerous biological processes, including viral infection. Here, we show that m6A and its associated machinery regulate the life cycle of hepatitis B virus (HBV). HBV is a DNA virus that completes its life cycle via an RNA intermediate, termed pregenomic RNA (pgRNA). Silencing of enzymes that catalyze the addition of m6A to RNA resulted in increased HBV protein expression, but overall reduced reverse transcription of the pgRNA. We mapped the m6A site in the HBV RNA and found that a conserved m6A consensus motif situated within the epsilon stem loop structure, is the site for m6A modification. The epsilon stem loop is located in the 3' terminus of all HBV mRNAs and at both the 5' and 3' termini of the pgRNA. Mutational analysis of the identified m6A site in the 5' epsilon stem loop of pgRNA revealed that m6A at this site is required for efficient reverse transcription of pgRNA, while m6A methylation of the 3' epsilon stem loop results in destabilization of all HBV transcripts, suggesting that m6A has dual regulatory function for HBV RNA. Overall, this study reveals molecular insights into how m6A regulates HBV gene expression and reverse transcription, leading to an increased level of understanding of the HBV life cycle.
Keywords: HBV reverse transcription; RNA methylation; epsilon loop; hepatitis B virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

