Biophysical and functional characterization of Norrin signaling through Frizzled4
- PMID: 30104375
- PMCID: PMC6126767
- DOI: 10.1073/pnas.1805901115
Biophysical and functional characterization of Norrin signaling through Frizzled4
Erratum in
-
Correction for Bang et al., Biophysical and functional characterization of Norrin signaling through Frizzled4.Proc Natl Acad Sci U S A. 2018 Nov 6;115(45):E10807. doi: 10.1073/pnas.1817333115. Epub 2018 Oct 29. Proc Natl Acad Sci U S A. 2018. PMID: 30373841 Free PMC article. No abstract available.
Abstract
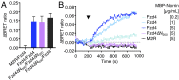
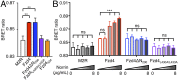
Wnt signaling is initiated by Wnt ligand binding to the extracellular ligand binding domain, called the cysteine-rich domain (CRD), of a Frizzled (Fzd) receptor. Norrin, an atypical Fzd ligand, specifically interacts with Fzd4 to activate β-catenin-dependent canonical Wnt signaling. Much of the molecular basis that confers Norrin selectivity in binding to Fzd4 was revealed through the structural study of the Fzd4CRD-Norrin complex. However, how the ligand interaction, seemingly localized at the CRD, is transmitted across full-length Fzd4 to the cytoplasm remains largely unknown. Here, we show that a flexible linker domain, which connects the CRD to the transmembrane domain, plays an important role in Norrin signaling. The linker domain directly contributes to the high-affinity interaction between Fzd4 and Norrin as shown by ∼10-fold higher binding affinity of Fzd4CRD to Norrin in the presence of the linker. Swapping the Fzd4 linker with the Fzd5 linker resulted in the loss of Norrin signaling, suggesting the importance of the linker in ligand-specific cellular response. In addition, structural dynamics of Fzd4 associated with Norrin binding investigated by hydrogen/deuterium exchange MS revealed Norrin-induced conformational changes on the linker domain and the intracellular loop 3 (ICL3) region of Fzd4. Cell-based functional assays showed that linker deletion, L430A and L433A mutations at ICL3, and C-terminal tail truncation displayed reduced β-catenin-dependent signaling activity, indicating the functional significance of these sites. Together, our results provide functional and biochemical dissection of Fzd4 in Norrin signaling.
Keywords: Dishevelled; Frizzled 4; Norrin; linker domain; signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. - PubMed
-
- Dann CE, et al. Insights into Wnt binding and signalling from the structures of two Frizzled cysteine-rich domains. Nature. 2001;412:86–90. - PubMed
-
- Berger W. Molecular dissection of Norrie disease. Acta Anat (Basel) 1998;162:95–100. - PubMed
-
- Xu Q, et al. Vascular development in the retina and inner ear: Control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116:883–895. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

