Interleukin 4 is inactivated via selective disulfide-bond reduction by extracellular thioredoxin
- PMID: 30104382
- PMCID: PMC6126747
- DOI: 10.1073/pnas.1805288115
Interleukin 4 is inactivated via selective disulfide-bond reduction by extracellular thioredoxin
Abstract
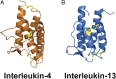
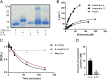
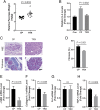
Thioredoxin 1 (TRX), an essential intracellular redox regulator, is also secreted by mammalian cells. Recently, we showed that TRX activates extracellular transglutaminase 2 via reduction of an allosteric disulfide bond. In an effort to identify other extracellular substrates of TRX, macrophages derived from THP-1 cells were treated with NP161, a small-molecule inhibitor of secreted TRX. NP161 enhanced cytokine outputs of alternatively activated macrophages, suggesting that extracellular TRX regulated the activity of interleukin 4 (IL-4) and/or interleukin 13 (IL-13). To test this hypothesis, the C35S mutant of human TRX was shown to form a mixed disulfide bond with recombinant IL-4 but not IL-13. Kinetic analysis revealed a kcat/KM value of 8.1 μM-1⋅min-1 for TRX-mediated recognition of IL-4, which established this cytokine as the most selective partner of extracellular TRX to date. Mass spectrometry identified the C46-C99 bond of IL-4 as the target of TRX, consistent with the essential role of this disulfide bond in IL-4 activity. To demonstrate the physiological relevance of our biochemical findings, recombinant TRX was shown to attenuate IL-4-dependent proliferation of cultured TF-1 erythroleukemia cells and also to inhibit the progression of chronic pancreatitis in an IL-4-driven mouse model of this disease. By establishing that IL-4 is posttranslationally regulated by TRX-promoted reduction of a disulfide bond, our findings highlight a novel regulatory mechanism of the type 2 immune response that is specific to IL-4 over IL-13.
Keywords: M2; disulfide bond; interleukin 4; macrophages; thioredoxin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Thioredoxin-1 Selectively Activates Transglutaminase 2 in the Extracellular Matrix of the Small Intestine: IMPLICATIONS FOR CELIAC DISEASE.J Biol Chem. 2017 Feb 3;292(5):2000-2008. doi: 10.1074/jbc.M116.767988. Epub 2016 Dec 21. J Biol Chem. 2017. PMID: 28003361 Free PMC article.
-
Activation of extracellular transglutaminase 2 by thioredoxin.J Biol Chem. 2011 Oct 28;286(43):37866-73. doi: 10.1074/jbc.M111.287490. Epub 2011 Sep 9. J Biol Chem. 2011. PMID: 21908620 Free PMC article.
-
Regulation of interleukin-4 signaling by extracellular reduction of intramolecular disulfides.Biochem Biophys Res Commun. 2009 Dec 25;390(4):1272-7. doi: 10.1016/j.bbrc.2009.10.134. Epub 2009 Oct 28. Biochem Biophys Res Commun. 2009. PMID: 19878651
-
Redox regulation of cellular activation.Annu Rev Immunol. 1997;15:351-69. doi: 10.1146/annurev.immunol.15.1.351. Annu Rev Immunol. 1997. PMID: 9143692 Review.
-
Conferring specificity in redox pathways by enzymatic thiol/disulfide exchange reactions.Free Radic Res. 2016;50(2):206-45. doi: 10.3109/10715762.2015.1120864. Free Radic Res. 2016. PMID: 26573728 Review.
Cited by
-
Autonomous Non Antioxidant Roles for Fasciola hepatica Secreted Thioredoxin-1 and Peroxiredoxin-1.Front Cell Infect Microbiol. 2021 May 5;11:667272. doi: 10.3389/fcimb.2021.667272. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34026663 Free PMC article. Review.
-
Allosteric disulphide bonds as reversible mechano-sensitive switches that control protein functions in the vasculature.Biophys Rev. 2019 Jun;11(3):419-430. doi: 10.1007/s12551-019-00543-0. Epub 2019 May 14. Biophys Rev. 2019. PMID: 31090016 Free PMC article. Review.
-
Harnessing the intrinsic photochemistry of isoxazoles for the development of chemoproteomic crosslinking methods.Chem Commun (Camb). 2022 Aug 11;58(65):9116-9119. doi: 10.1039/d2cc02263j. Chem Commun (Camb). 2022. PMID: 35880535 Free PMC article.
-
Allosteric disulfides: Sophisticated molecular structures enabling flexible protein regulation.J Biol Chem. 2019 Feb 22;294(8):2949-2960. doi: 10.1074/jbc.REV118.005604. Epub 2019 Jan 10. J Biol Chem. 2019. PMID: 30635401 Free PMC article. Review.
-
Human and Bacterial Toll-Interleukin Receptor Domains Exhibit Distinct Dynamic Features and Functions.Molecules. 2022 Jul 14;27(14):4494. doi: 10.3390/molecules27144494. Molecules. 2022. PMID: 35889366 Free PMC article.
References
-
- Holmgren A, Lu J. Thioredoxin and thioredoxin reductase: Current research with special reference to human disease. Biochem Biophys Res Commun. 2010;396:120–124. - PubMed
-
- Nakamura H. Thioredoxin as a key molecule in redox signaling. Antioxid Redox Signal. 2004;6:15–17. - PubMed
-
- Rubartelli A, Bajetto A, Allavena G, Wollman E, Sitia R. Secretion of thioredoxin by normal and neoplastic cells through a leaderless secretory pathway. J Biol Chem. 1992;267:24161–24164. - PubMed
-
- Lillig CH, Holmgren A. Thioredoxin and related molecules—From biology to health and disease. Antioxid Redox Signal. 2007;9:25–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

