Deciphering the super relaxed state of human β-cardiac myosin and the mode of action of mavacamten from myosin molecules to muscle fibers
- PMID: 30104387
- PMCID: PMC6126717
- DOI: 10.1073/pnas.1809540115
Deciphering the super relaxed state of human β-cardiac myosin and the mode of action of mavacamten from myosin molecules to muscle fibers
Abstract
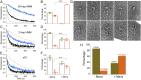
Mutations in β-cardiac myosin, the predominant motor protein for human heart contraction, can alter power output and cause cardiomyopathy. However, measurements of the intrinsic force, velocity, and ATPase activity of myosin have not provided a consistent mechanism to link mutations to muscle pathology. An alternative model posits that mutations in myosin affect the stability of a sequestered, super relaxed state (SRX) of the protein with very slow ATP hydrolysis and thereby change the number of myosin heads accessible to actin. Here we show that purified human β-cardiac myosin exists partly in an SRX and may in part correspond to a folded-back conformation of myosin heads observed in muscle fibers around the thick filament backbone. Mutations that cause hypertrophic cardiomyopathy destabilize this state, while the small molecule mavacamten promotes it. These findings provide a biochemical and structural link between the genetics and physiology of cardiomyopathy with implications for therapeutic strategies.
Keywords: cardiac inhibitor; interacting heads motif; mavacamten; myosin; super relaxed state.
Conflict of interest statement
Conflict of interest statement: J.A.S. is a cofounder of MyoKardia, a biotechnology company developing small molecules that target the sarcomere for the treatment of inherited cardiomyopathies, and of Cytokinetics and is a member of their scientific advisory boards. J.G.S. is a cofounder of MyoKardia and a member of its scientific advisory board. K.M.R. and R.C. are members of the MyoKardia scientific advisory board. R.L.A., M.H., and F.L.W. are employees of and own shares in MyoKardia. E.M.G. owns shares in MyoKardia.
Figures
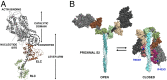




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

