Targeted Therapies in Type II Endometrial Cancers: Too Little, but Not Too Late
- PMID: 30104481
- PMCID: PMC6121653
- DOI: 10.3390/ijms19082380
Targeted Therapies in Type II Endometrial Cancers: Too Little, but Not Too Late
Abstract
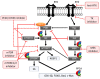
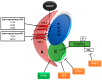
Type II endometrial carcinomas (ECs) are responsible for most endometrial cancer-related deaths due to their aggressive nature, late stage detection and high tolerance for standard therapies. However, there are no targeted therapies for type II ECs, and they are still treated the same way as the clinically indolent and easily treatable type I ECs. Therefore, type II ECs are in need of new treatment options. More recently, molecular analysis of endometrial cancer revealed phosphorylation-dependent oncogenic signalling in the phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) pathways to be most frequently altered in type II ECs. Consequently, clinical trials tested pharmacologic kinase inhibitors targeting these pathways, although mostly with rather disappointing results. In this review, we highlight the most common genetic alterations in type II ECs. Additionally, we reason why most clinical trials for ECs using targeted kinase inhibitors had unsatisfying results and what should be changed in future clinical trial setups. Furthermore, we argue that, besides kinases, phosphatases should no longer be ignored in clinical trials, particularly in type II ECs, where the tumour suppressive phosphatase protein phosphatase type 2A (PP2A) is frequently mutated. Lastly, we discuss the therapeutic potential of targeting PP2A for (re)activation, possibly in combination with pharmacologic kinase inhibitors.
Keywords: PP2A; PPP2R1A; SMAP; endometrial cancer; kinase inhibitor; molecular marker; protein kinase; protein phosphatase; targeted therapy; type II endometrial carcinoma.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, or in the writing of the manuscript.
Figures
References
-
- World Health Organization . Global Health Estimates 2015: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2015. World Health Organization; Geneva, Switzerland: 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

