Synthesis and Preliminary Evaluation of Biological Activity of Glycoconjugates Analogues of Acyclic Uridine Derivatives
- PMID: 30104510
- PMCID: PMC6222857
- DOI: 10.3390/molecules23082017
Synthesis and Preliminary Evaluation of Biological Activity of Glycoconjugates Analogues of Acyclic Uridine Derivatives
Abstract
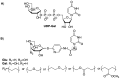
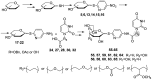
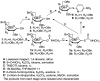
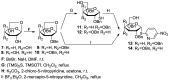
Herein we present the methodology for obtaining glycosyltransferase inhibitors, analogues of natural enzyme substrates of donor-type: UDP-glucose and UDP-galactose. The synthesis concerned glycoconjugates, nucleoside analogues containing an acyclic ribose mimetic linked to a uracil moiety in their structure. The biological activity of the synthesised compounds was determined on the basis of their ability to inhibit the model enzyme action of β-1,4-galactosyltransferase from bovine milk. The obtained results allowed to expand and supplement the existing library of synthetic compounds that are able to regulate the biological activity of enzymes from the GT class.
Keywords: acyclic uridine derivatives; glycoconjugates; glycosyltransferase inhibitors; glycosyltransferases; thioglycosides.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Glycoconjugates, products of uridine derivatives phosphitylation and oxidation as glycosyltransferases potential inhibitors.Acta Pol Pharm. 2010 Nov-Dec;67(6):652-63. Acta Pol Pharm. 2010. PMID: 21229882
-
Synthesis and preliminary biological assay of uridine glycoconjugate derivatives containing amide and/or 1,2,3-triazole linkers.Bioorg Chem. 2017 Jun;72:80-88. doi: 10.1016/j.bioorg.2017.03.015. Epub 2017 Mar 30. Bioorg Chem. 2017. PMID: 28384485
-
Synthesis of fucosylated uridine conjugates as potential glycosyltransferase inhibitors.Acta Pol Pharm. 2014 Nov-Dec;71(6):1083-9. Acta Pol Pharm. 2014. PMID: 25745784 No abstract available.
-
Iminosugars as glycosyltransferase inhibitors.Org Biomol Chem. 2021 Jun 30;19(25):5439-5475. doi: 10.1039/d1ob00382h. Org Biomol Chem. 2021. PMID: 33881114 Review.
-
Design, synthesis and biological evaluation of iminosugar-based glycosyltransferase inhibitors.Curr Top Med Chem. 2003;3(5):541-60. doi: 10.2174/1568026033452474. Curr Top Med Chem. 2003. PMID: 12570865 Review.
Cited by
-
Design, Synthesis, and Evaluation of Novel 2-Methoxyestradiol Derivatives as Apoptotic Inducers Through an Intrinsic Apoptosis Pathway.Biomolecules. 2020 Jan 10;10(1):123. doi: 10.3390/biom10010123. Biomolecules. 2020. PMID: 31936880 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

