Cooperativity in Plant Plasma Membrane Intrinsic Proteins (PIPs): Mechanism of Increased Water Transport in Maize PIP1 Channels in Hetero-tetramers
- PMID: 30104609
- PMCID: PMC6089885
- DOI: 10.1038/s41598-018-30257-4
Cooperativity in Plant Plasma Membrane Intrinsic Proteins (PIPs): Mechanism of Increased Water Transport in Maize PIP1 Channels in Hetero-tetramers
Abstract
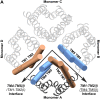
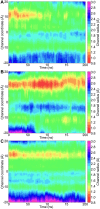
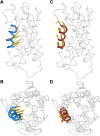
Plant aquaporins (AQPs) play vital roles in several physiological processes. Plasma membrane intrinsic proteins (PIPs) belong to the subfamily of plant AQPs. They are further subdivided into two closely related subgroups PIP1s and PIP2s. While PIP2 members are efficient water channels, PIP1s from some plant species have been shown to be functionally inactive. Aquaporins form tetramers under physiological conditions. PIP2s can enhance the water transport of PIP1s when they form hetero-tetramers. However, the role of monomer-monomer interface and the significance of specific residues in enhancing the water permeation of PIP1s have not been investigated at atomic level. We have performed all-atom molecular dynamics (MD) simulations of homo-tetramers and four different hetero-tetramers containing ZmPIP1;2 and ZmPIP2;5 from Zea mays. ZmPIP1;2 in a tetramer assembly will have two interfaces, one formed by transmembrane segments TM4 and TM5 and the other formed by TM1 and TM2. We have analyzed channel radius profiles, water transport and potential of mean force profiles of ZmPIP1;2 monomers. Results of MD simulations clearly revealed the influence of TM4-TM5 interface in modulating the water transport of ZmPIP1;2. MD simulations indicate the importance of I93 residue from the TM2 segment of ZmPIP2;5 for the increased water transport in ZmPIP1;2.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

