Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches
- PMID: 30104732
- PMCID: PMC6342021
- DOI: 10.1038/s41593-018-0203-4
Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches
Abstract
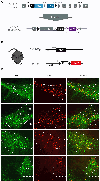
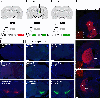
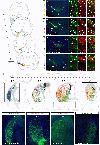
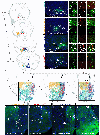
Midbrain dopamine (DA) neurons have diverse functions that can in part be explained by their heterogeneity. Although molecularly distinct subtypes of DA neurons have been identified by single-cell gene expression profiling, fundamental features such as their projection patterns have not been elucidated. Progress in this regard has been hindered by the lack of genetic tools for studying DA neuron subtypes. Here we develop intersectional genetic labeling strategies, based on combinatorial gene expression, to map the projections of molecularly defined DA neuron subtypes. We reveal distinct genetically defined dopaminergic pathways arising from the substantia nigra pars compacta and from the ventral tegmental area that innervate specific regions of the caudate putamen, nucleus accumbens and amygdala. Together, the genetic toolbox and DA neuron subtype projections presented here constitute a resource that will accelerate the investigation of this clinically significant neurotransmitter system.
Figures
Similar articles
-
Dopamine-glutamate neuron projections to the nucleus accumbens medial shell and behavioral switching.Neurochem Int. 2019 Oct;129:104482. doi: 10.1016/j.neuint.2019.104482. Epub 2019 Jun 3. Neurochem Int. 2019. PMID: 31170424 Free PMC article. Review.
-
Forebrain projections from cholecystokininlike-immunoreactive neurons in the rat midbrain.J Comp Neurol. 1989 Jan 15;279(3):415-35. doi: 10.1002/cne.902790307. J Comp Neurol. 1989. PMID: 2918078
-
Biochemical mapping of the noradrenergic ventral bundle projection sites: evidence for a noradrenergic--dopaminergic interaction.Brain Res. 1979 Aug 17;172(1):87-100. doi: 10.1016/0006-8993(79)90897-7. Brain Res. 1979. PMID: 466469
-
In vivo functional diversity of midbrain dopamine neurons within identified axonal projections.Elife. 2019 Oct 3;8:e48408. doi: 10.7554/eLife.48408. Elife. 2019. PMID: 31580257 Free PMC article.
-
Molecular heterogeneity in the substantia nigra: A roadmap for understanding PD motor pathophysiology.Neurobiol Dis. 2022 Dec;175:105925. doi: 10.1016/j.nbd.2022.105925. Epub 2022 Nov 11. Neurobiol Dis. 2022. PMID: 36372290 Review.
Cited by
-
A hindbrain dopaminergic neural circuit prevents weight gain by reinforcing food satiation.Sci Adv. 2021 May 26;7(22):eabf8719. doi: 10.1126/sciadv.abf8719. Print 2021 May. Sci Adv. 2021. PMID: 34039606 Free PMC article.
-
Behaviorally penetrant, anomalous dopamine efflux exposes sex and circuit dependent regulation of dopamine transporters.Mol Psychiatry. 2022 Dec;27(12):4869-4880. doi: 10.1038/s41380-022-01773-7. Epub 2022 Sep 18. Mol Psychiatry. 2022. PMID: 36117213
-
Midbrain Dopaminergic Neuron Development at the Single Cell Level: In vivo and in Stem Cells.Front Cell Dev Biol. 2020 Jun 25;8:463. doi: 10.3389/fcell.2020.00463. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32733875 Free PMC article. Review.
-
D1 receptor-expressing neurons in ventral tegmental area alleviate mouse anxiety-like behaviors via glutamatergic projection to lateral septum.Mol Psychiatry. 2023 Feb;28(2):625-638. doi: 10.1038/s41380-022-01809-y. Epub 2022 Oct 4. Mol Psychiatry. 2023. PMID: 36195641 Free PMC article.
-
Biophysical Modeling of Dopaminergic Denervation Landscapes in the Striatum Reveals New Therapeutic Strategy.eNeuro. 2022 Mar 3;9(2):ENEURO.0458-21.2022. doi: 10.1523/ENEURO.0458-21.2022. Print 2022 Mar-Apr. eNeuro. 2022. PMID: 35165198 Free PMC article.
References
-
- Björklund AA & Dunnett SB Dopamine neuron systems in the brain: an update. Trends Neurosci 30, 194–202 (2007). - PubMed
-
- Roeper J Dissecting the diversity of midbrain dopamine neurons. Trends Neurosci 36, 336–342 (2013). - PubMed
-
- Morales M & Margolis EB Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci 18, 73–85 (2017). - PubMed
Methods reference list
-
- Donnelly ML et al. Analysis of the aphthovirus 2A/2B polyprotein ‘cleavage’ mechanism indicates not a proteolytic reaction, but a novel translational effect: a putative ribosomal ‘skip’. J. Gen. Virol 82, 1013–1025 (2001). - PubMed
-
- Anastassiadis K et al. Dre recombinase, like Cre, is a highly efficient site-specific recombinase in E. coli, mammalian cells and mice. Dis Model Mech 2, 508–515 (2009). - PubMed
-
- Wallén A et al. Fate of mesencephalic AHD2-expressing dopamine progenitor cells in NURR1 mutant mice. Exp. Cell Res. 253, 737–746 (1999). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

