Voluntary urination control by brainstem neurons that relax the urethral sphincter
- PMID: 30104734
- PMCID: PMC6119086
- DOI: 10.1038/s41593-018-0204-3
Voluntary urination control by brainstem neurons that relax the urethral sphincter
Abstract
Voluntary urination ensures that waste is eliminated when safe and socially appropriate, even without a pressing urge. Uncontrolled urination, or incontinence, is a common problem with few treatment options. Normal urine release requires a small region in the brainstem known as Barrington's nucleus (Bar), but specific neurons that relax the urethral sphincter and enable urine flow are unknown. Here we identify a small subset of Bar neurons that control the urethral sphincter in mice. These excitatory neurons express estrogen receptor 1 (BarESR1), project to sphincter-relaxing interneurons in the spinal cord and are active during natural urination. Optogenetic stimulation of BarESR1 neurons rapidly initiates sphincter bursting and efficient voiding in anesthetized and behaving animals. Conversely, optogenetic and chemogenetic inhibition reveals their necessity in motivated urination behavior. The identification of these cells provides an expanded model for the control of urination and its dysfunction.
Conflict of interest statement
The authors declare no competing interests.
Figures

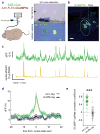
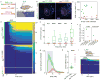


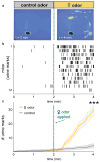

Comment in
-
Bar neurons and urination.Nat Rev Urol. 2018 Nov;15(11):658. doi: 10.1038/s41585-018-0078-x. Nat Rev Urol. 2018. PMID: 30131601 No abstract available.
-
Let it go: central neural control of urination.Nat Neurosci. 2018 Nov;21(11):1499-1501. doi: 10.1038/s41593-018-0259-1. Nat Neurosci. 2018. PMID: 30361546 No abstract available.
References
-
- Holstege G. The emotional motor system and micturition control. Neurourol Urodyn. 2010;29:42–48. - PubMed
-
- Minassian VA, Drutz HP, Al-Badr A. Urinary incontinence as a worldwide problem. Int J Gynecol Obstet. 2003;82:327–338. - PubMed
-
- Bayani D-M, Taborsky M, Frommen JG. To pee or not to pee: urine signals mediate aggressive interactions in the cooperatively breeding cichlid Neolamprologus pulcher. Behav Ecol Sociobiol. 2017;71
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

