Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors
- PMID: 30104766
- PMCID: PMC6129206
- DOI: 10.1038/s41591-018-0135-2
Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors
Erratum in
-
Author Correction: Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors.Nat Med. 2019 Mar;25(3):529. doi: 10.1038/s41591-019-0355-0. Nat Med. 2019. PMID: 30670876 Free PMC article.
Abstract
T cell dysfunction contributes to tumor immune escape in patients with cancer and is particularly severe amidst glioblastoma (GBM). Among other defects, T cell lymphopenia is characteristic, yet often attributed to treatment. We reveal that even treatment-naïve subjects and mice with GBM can harbor AIDS-level CD4 counts, as well as contracted, T cell-deficient lymphoid organs. Missing naïve T cells are instead found sequestered in large numbers in the bone marrow. This phenomenon characterizes not only GBM but a variety of other cancers, although only when tumors are introduced into the intracranial compartment. T cell sequestration is accompanied by tumor-imposed loss of S1P1 from the T cell surface and is reversible upon precluding S1P1 internalization. In murine models of GBM, hindering S1P1 internalization and reversing sequestration licenses T cell-activating therapies that were previously ineffective. Sequestration of T cells in bone marrow is therefore a tumor-adaptive mode of T cell dysfunction, whose reversal may constitute a promising immunotherapeutic adjunct.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
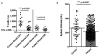
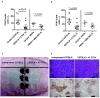


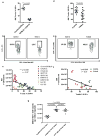
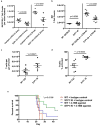
Comment in
-
With GBM, T Cells May Be Stuck in Bone Marrow.Cancer Discov. 2018 Oct;8(10):1203-1204. doi: 10.1158/2159-8290.CD-NB2018-117. Epub 2018 Sep 5. Cancer Discov. 2018. PMID: 30185625
-
Where Have All the T Cells Gone?Immunity. 2018 Oct 16;49(4):592-594. doi: 10.1016/j.immuni.2018.10.006. Immunity. 2018. PMID: 30332627 Free PMC article.
-
Missing Naïve T Cells in the Setting of Glioblastoma Are Found to Be Sequestered in the Bone Marrow.Neurosurgery. 2019 Aug 1;85(2):E194-E195. doi: 10.1093/neuros/nyz167. Neurosurgery. 2019. PMID: 31304546 No abstract available.
References
-
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 2002;3:991–998. - PubMed
-
- Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. - PubMed
-
- Dix AR, Brooks WH, Roszman TL, Morford LA. Immune defects observed in patients with primary malignant brain tumors. J. Neuroimmunol. 1999;100:216–232. - PubMed
-
- Dunn GP, Fecci PE, Curry WT. Cancer immunoediting in malignant glioma. Neurosurgery. 2012;71:201–222. discussion 222-203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

