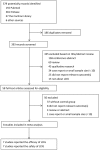
Levodopa-Carbidopa Intestinal Gel in Parkinson's Disease: A Systematic Review and Meta-Analysis
- PMID: 30104997
- PMCID: PMC6077236
- DOI: 10.3389/fneur.2018.00620
Levodopa-Carbidopa Intestinal Gel in Parkinson's Disease: A Systematic Review and Meta-Analysis
Abstract
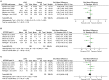
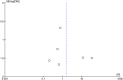
Background: Levodopa has been widely used and regarded as the most effective therapy for Parkinson's disease (PD), but long-term treatment with oral levodopa may result in motor fluctuations and involuntary movements (dyskinesias). There is evidence to suggest that Continuous infusion of levodopa-carbidopa intestinal gel (LCIG) can effectively manage motor and non-motor complications in PD, but clinical studies investigating this have yielded inconsistent results. This systematic review and meta-analysis was performed to examine the efficacy and safety of LCIG for patients with PD. Methods: A systematic search was conducted to retrieve published data in the EMBASE, PubMed, and the Cochrane Library up to March 2018. Both efficiency and safety of LCIG were analyzed using pooled standardized mean differences (SMDs) or odds ratio (ORs) with 95% confidence interval (CIs). Results: Eight trials with 384 PD patients were included in the present study. Compared with the control group, LCIG significantly decreased off-time (SMD, -1.19; 95% CI, -2.25 to -0.12; p = 0.003) and increased on-time without troublesome dyskinesia (SMD, 0.55; 95% CI, 0.20 to 0.90; p = 0.002). However, no significant difference of LCIG was found in on-time with troublesome dyskinesia. There were no significant differences in UPDRS, Hoehn & Yahr and PDQ-39 scores. Besides, no significant differences in the drop-out and adverse effects. Conclusions: Continuous delivery of LCIG may offer a promising option for PD patients. More randomized double-blind controlled studies with large sample sizes were needed to further confirm the efficacy and safety of LCIG for PD patients.
Keywords: Parkinson's disease; efficacy; levodopa-carbidopa intestinal gel; meta-analysis; safety.
Figures




Similar articles
-
Comparison Between Levodopa-Carbidopa Intestinal Gel Infusion and Subthalamic Nucleus Deep-Brain Stimulation for Advanced Parkinson's Disease: A Systematic Review and Meta-Analysis.Front Neurol. 2019 Aug 27;10:934. doi: 10.3389/fneur.2019.00934. eCollection 2019. Front Neurol. 2019. PMID: 31507529 Free PMC article.
-
Jejunal Infusion of levodopa-carbidopa intestinal gel versus oral administration of levodopa-carbidopa tablets in japanese subjects with advanced Parkinson's disease: pharmacokinetics and pilot efficacy and safety.Clin Pharmacokinet. 2015 Sep;54(9):975-84. doi: 10.1007/s40262-015-0265-3. Clin Pharmacokinet. 2015. PMID: 25875940 Free PMC article. Clinical Trial.
-
Levodopa-Carbidopa Intestinal Gel Monotherapy: GLORIA Registry Demographics, Efficacy, and Safety.J Parkinsons Dis. 2019;9(3):531-541. doi: 10.3233/JPD-191605. J Parkinsons Dis. 2019. PMID: 31282424 Free PMC article.
-
The effect of levodopa-carbidopa intestinal gel infusion long-term therapy on motor complications in advanced Parkinson's disease: a multicenter Romanian experience.J Neural Transm (Vienna). 2016 Apr;123(4):407-14. doi: 10.1007/s00702-015-1496-z. Epub 2015 Dec 23. J Neural Transm (Vienna). 2016. PMID: 26699635 Free PMC article.
-
Levodopa-carbidopa enteral suspension in advanced Parkinson's disease: clinical evidence and experience.Ther Adv Neurol Disord. 2017 Mar;10(3):171-187. doi: 10.1177/1756285616681280. Epub 2016 Dec 1. Ther Adv Neurol Disord. 2017. PMID: 28344656 Free PMC article. Review.
Cited by
-
Concomitant Medication Usage with Levodopa-Carbidopa Intestinal Gel: Results from the COSMOS Study.Mov Disord. 2021 Aug;36(8):1853-1862. doi: 10.1002/mds.28596. Epub 2021 Apr 28. Mov Disord. 2021. PMID: 33908647 Free PMC article.
-
Cognitive Outcomes of Advanced Therapies in Parkinson's Disease: A Systematic Review of Apomorphine and Levodopa-Carbidopa Intestinal Gel Therapies.Eur J Neurol. 2025 Feb;32(2):e70077. doi: 10.1111/ene.70077. Eur J Neurol. 2025. PMID: 39932029 Free PMC article.
-
An Update on Medical and Surgical Treatments of Parkinson's Disease.Aging Dis. 2021 Jul 1;12(4):1021-1035. doi: 10.14336/AD.2020.1225. eCollection 2021 Jul. Aging Dis. 2021. PMID: 34221546 Free PMC article. Review.
-
24-Hour Levodopa-Carbidopa Intestinal Gel: Clinical Experience and Practical Recommendations.CNS Drugs. 2021 Feb;35(2):137-149. doi: 10.1007/s40263-020-00782-w. Epub 2021 Feb 13. CNS Drugs. 2021. PMID: 33582982 Free PMC article. Review.
-
Stem Cell-based and Advanced Therapeutic Modalities for Parkinson's Disease: A Risk-effectiveness Patient-centered Analysis.Curr Neuropharmacol. 2022 Nov 15;20(12):2320-2345. doi: 10.2174/1570159X20666220201100238. Curr Neuropharmacol. 2022. PMID: 35105291 Free PMC article. Review.
References
-
- Olanow C W., Watts R. L., Koller W. C. (2001). An algorithm (decision tree) for the management of Parkinson's disease: treatment guidelines. Neurology (2001) 56(11 Suppl. 5):S1–88. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

