The Thomsen-Friedenreich Antigen-Specific Antibody Signatures in Patients with Breast Cancer
- PMID: 30105268
- PMCID: PMC6076901
- DOI: 10.1155/2018/9579828
The Thomsen-Friedenreich Antigen-Specific Antibody Signatures in Patients with Breast Cancer
Abstract
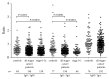
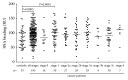
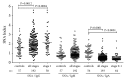
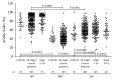
Alterations in the glycosylation of serum total immunoglobulins show these antibodies to have a diagnostic potential for cancer but the disease-related Abs to the tumor-associated antigens, including glycans, have still poorly been investigated in this respect. We analysed serum samples from patients with breast carcinoma (n = 196) and controls (n = 64) for the level of Thomsen-Friedenreich (TF) antigen-specific antibody isotypes, their sialylation, interrelationships, and the avidity by using ELISA with the synthetic TF-polyacrylamide conjugate as an antigen and the sialic acid-specific Sambucus nigra agglutinin (SNA) and ammonium thiocyanate as a chaotrope. An increased sialylation of IgG and IgM, but a lower SNA reactivity of IgA TF antibodies, and a higher level and avidity of the TF-specific IgA were found in cancer patients. Other cancer-related signatures were the highly significant increase of the IgG/IgA ratio and the very low SNA/IgA index in cancer, including patients with an early stage of the disease. These changes showed a good diagnostic potential with about 80% accuracy. Thus, the level of naturally occurring anti-TF antigen antibodies, their sialylation profile, isotype distribution, and avidity displayed cancer-specific changes that could serve as novel noninvasive Ab-based biomarkers for early breast cancer.
Figures


Similar articles
-
Signatures of anti-Thomsen - Friedenreich antigen antibody diversity in colon cancer patients.Exp Oncol. 2018 Mar;40(1):48-58. Exp Oncol. 2018. PMID: 29600973
-
Increased Avidity of the Sambucus nigra Lectin-Reactive Antibodies to the Thomsen-Friedenreich Antigen as a Potential Biomarker for Gastric Cancer.Dis Markers. 2015;2015:761908. doi: 10.1155/2015/761908. Epub 2015 Nov 18. Dis Markers. 2015. PMID: 26663951 Free PMC article.
-
Increased sialylation of anti-Thomsen-Friedenreich antigen (CD176) antibodies in patients with gastric cancer: a diagnostic and prognostic potential.Biomed Res Int. 2014;2014:830847. doi: 10.1155/2014/830847. Epub 2014 Sep 4. Biomed Res Int. 2014. PMID: 25276822 Free PMC article.
-
Profiling of Naturally Occurring Antibodies to the Thomsen-Friedenreich Antigen in Health and Cancer: The Diversity and Clinical Potential.Biomed Res Int. 2020 Mar 23;2020:9747040. doi: 10.1155/2020/9747040. eCollection 2020. Biomed Res Int. 2020. PMID: 32280709 Free PMC article. Review.
-
The Thomsen-Friedenreich (TF) antigen: a critical review on the structural, biosynthetic and histochemical aspects of a pancarcinoma-associated antigen.Histol Histopathol. 1997 Jan;12(1):263-81. Histol Histopathol. 1997. PMID: 9046061 Review.
Cited by
-
Can serum autoantibodies be a potential early detection biomarker for breast cancer in women? A diagnostic test accuracy review and meta-analysis.Syst Rev. 2022 Oct 9;11(1):215. doi: 10.1186/s13643-022-02088-y. Syst Rev. 2022. PMID: 36210467 Free PMC article. Review.
-
B cell heterogeneity, plasticity, and functional diversity in cancer microenvironments.Oncogene. 2021 Jul;40(29):4737-4745. doi: 10.1038/s41388-021-01918-y. Epub 2021 Jun 29. Oncogene. 2021. PMID: 34188249 Review.
-
Advances in IgA glycosylation and its correlation with diseases.Front Chem. 2022 Sep 27;10:974854. doi: 10.3389/fchem.2022.974854. eCollection 2022. Front Chem. 2022. PMID: 36238099 Free PMC article. Review.
-
The Dual Role of B Cells in the Tumor Microenvironment: Implications for Cancer Immunology and Therapy.Int J Mol Sci. 2024 Nov 4;25(21):11825. doi: 10.3390/ijms252111825. Int J Mol Sci. 2024. PMID: 39519376 Free PMC article. Review.
-
Identification and Targeting of Thomsen-Friedenreich and IL1RAP Antigens on Chronic Myeloid Leukemia Stem Cells Using Bi-Specific Antibodies.Onco Targets Ther. 2021 Jan 22;14:609-621. doi: 10.2147/OTT.S255299. eCollection 2021. Onco Targets Ther. 2021. PMID: 33519209 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

