Increased Axonal Bouton Stability during Learning in the Mouse Model of MECP2 Duplication Syndrome
- PMID: 30105297
- PMCID: PMC6086213
- DOI: 10.1523/ENEURO.0056-17.2018
Increased Axonal Bouton Stability during Learning in the Mouse Model of MECP2 Duplication Syndrome
Abstract
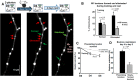
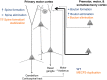
MECP2 duplication syndrome is an X-linked form of syndromic autism caused by genomic duplication of the region encoding methyl-CpG-binding protein 2 (MECP2). Mice overexpressing MECP2 demonstrate social impairment, behavioral inflexibility, and altered patterns of learning and memory. Previous work showed abnormally increased stability of dendritic spines formed during motor training in the apical tuft of primary motor cortex (area M1) corticospinal neurons in the MECP2 duplication mouse model. In the current study, we measure the structural plasticity of axonal boutons in layer 5 pyramidal neuron projections to layer 1 of area M1 during motor training. In wild-type littermate control mice, we find that during rotarod training the bouton formation rate changes minimally, if at all, while the bouton elimination rate more than doubles. Notably, the observed upregulation in bouton elimination with training is absent in MECP2 duplication mice. This result provides further evidence of an imbalance between structural stability and plasticity in this form of syndromic autism. Furthermore, the observation that axonal bouton elimination more than doubles with motor training in wild-type animals contrasts with the increase of dendritic spine consolidation observed in corticospinal neurons at the same layer. This dissociation suggests that different area M1 microcircuits may manifest different patterns of structural synaptic plasticity during motor training.
Keywords: MECP2; autism; bouton; motor learning; plasticity; synaptic.
Figures


References
-
- Ash RT, Buffington SA, Park J, Costa-Mattioli M, Zoghbi HY, Smirnakis SM (2017) Excessive ERK-dependent synaptic clustering with enhanced motor learning in the MECP2 duplication syndrome mouse model of autism. bioRxiv. Advance online publication. Retrieved June 18, 2018. doi:10.1101/100875.
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
