Preserved cardiac function by vinculin enhances glucose oxidation and extends health- and life-span
- PMID: 30105314
- PMCID: PMC6086353
- DOI: 10.1063/1.5019592
Preserved cardiac function by vinculin enhances glucose oxidation and extends health- and life-span
Abstract
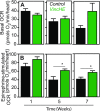
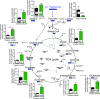
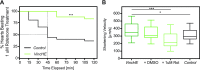
Despite limited regenerative capacity as we age, cardiomyocytes maintain their function in part through compensatory mechanisms, e.g., Vinculin reinforcement of intercalated discs in aged organisms. This mechanism, which is conserved from flies to non-human primates, creates a more crystalline sarcomere lattice that extends lifespan, but systemic connections between the cardiac sarcomere structure and lifespan extension are not apparent. Using the rapidly aging fly system, we found that cardiac-specific Vinculin-overexpression [Vinculin heart-enhanced (VincHE)] increases heart contractility, maximal cardiac mitochondrial respiration, and organismal fitness with age. Systemic metabolism also dramatically changed with age and VincHE; steady state sugar concentrations, as well as aerobic glucose metabolism, increase in VincHE and suggest enhanced energy substrate utilization with increased cardiac performance. When cardiac stress was induced with the complex I inhibitor rotenone, VincHE hearts sustain contractions unlike controls. This work establishes a new link between the cardiac cytoskeleton and systemic glucose utilization and protects mitochondrial function from external stress.
Figures


Similar articles
-
Vinculin network-mediated cytoskeletal remodeling regulates contractile function in the aging heart.Sci Transl Med. 2015 Jun 17;7(292):292ra99. doi: 10.1126/scitranslmed.aaa5843. Sci Transl Med. 2015. PMID: 26084806 Free PMC article.
-
Heterozygous inactivation of the vinculin gene predisposes to stress-induced cardiomyopathy.Am J Pathol. 2004 Sep;165(3):1033-44. doi: 10.1016/S0002-9440(10)63364-0. Am J Pathol. 2004. PMID: 15331426 Free PMC article.
-
Control of oxidative metabolism in volume-overloaded rat hearts: effect of propionyl-L-carnitine.Am J Physiol. 1997 Apr;272(4 Pt 2):H1615-24. doi: 10.1152/ajpheart.1997.272.4.H1615. Am J Physiol. 1997. PMID: 9139943
-
Contractile protein dynamics of myofibrils in paired adult rat cardiomyocytes.Cell Motil Cytoskeleton. 1993;26(4):301-12. doi: 10.1002/cm.970260405. Cell Motil Cytoskeleton. 1993. PMID: 8299146
-
Ketone bodies mimic the life span extending properties of caloric restriction.IUBMB Life. 2017 May;69(5):305-314. doi: 10.1002/iub.1627. Epub 2017 Apr 3. IUBMB Life. 2017. PMID: 28371201 Review.
Cited by
-
A practical guide for the analysis, standardization and interpretation of oxygen consumption measurements.Nat Metab. 2022 Aug;4(8):978-994. doi: 10.1038/s42255-022-00619-4. Epub 2022 Aug 15. Nat Metab. 2022. PMID: 35971004 Free PMC article. Review.
-
Bioengineering of the heart.APL Bioeng. 2020 Mar 4;4(1):010402. doi: 10.1063/1.5144525. eCollection 2020 Mar. APL Bioeng. 2020. PMID: 32161832 Free PMC article. No abstract available.
-
A Review of in vitro Platforms for Understanding Cardiomyocyte Mechanobiology.Front Bioeng Biotechnol. 2019 Jun 5;7:133. doi: 10.3389/fbioe.2019.00133. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31231644 Free PMC article. Review.
References
-
- Mozaffarian D., Benjamin E. J., Go A. S., Arnett D. K., Blaha M. J., Cushman M., Das S. R., de Ferranti S., Després J. P., Fullerton H. J., Howard V. J., Huffman M. D., Isasi C. R., Jiménez M. C., Judd S. E., Kissela B. M., Lichtman J. H., Lisabeth L. D., Liu S., Mackey R. H., Magid D. J., McGuire D. K., Mohler E. R., Moy C. S., Muntner P., Mussolino M. E., Nasir K., Neumar R. W., Nichol G., Palaniappan L., Pandey D. K., Reeves M. J., Rodriguez C. J., Rosamond W., Sorlie P. D., Stein J., Towfighi A., Turan T. N., Virani S. S., Woo D., Yeh R. W., Turner M. B., and Subcommittee AHASCaSS., “ Heart disease and stroke statistics-2016 update: A report from the American Heart Association,” Circulation 133(4), e38–e360 (2016).10.1161/CIR.0000000000000350 - DOI - PubMed
-
- Horn M. A., Graham H. K., Richards M. A., Clarke J. D., Greensmith D. J., Briston S. J., Hall M. C., Dibb K. M., and Trafford A. W., “ Age-related divergent remodeling of the cardiac extracellular matrix in heart failure: Collagen accumulation in the young and loss in the aged,” J. Mol. Cell Cardiol. 53(1), 82–90 (2012).10.1016/j.yjmcc.2012.03.011 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

