SLC2A9 (GLUT9) mediates urate reabsorption in the mouse kidney
- PMID: 30105595
- PMCID: PMC6224025
- DOI: 10.1007/s00424-018-2190-4
SLC2A9 (GLUT9) mediates urate reabsorption in the mouse kidney
Abstract
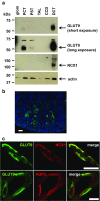
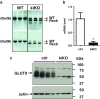
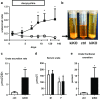
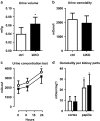
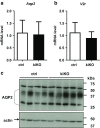
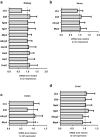
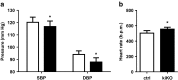
Uric acid (UA) is a metabolite of purine degradation and is involved in gout flairs and kidney stones formation. GLUT9 (SLC2A9) was previously shown to be a urate transporter in vitro. In vivo, humans carrying GLUT9 loss-of-function mutations have familial renal hypouricemia type 2, a condition characterized by hypouricemia, UA renal wasting associated with kidney stones, and an increased propensity to acute renal failure during strenuous exercise. Mice carrying a deletion of GLUT9 in the whole body are hyperuricemic and display a severe nephropathy due to intratubular uric acid precipitation. However, the precise role of GLUT9 in the kidney remains poorly characterized. We developed a mouse model in which GLUT9 was deleted specifically along the whole nephron in a tetracycline-inducible manner (subsequently called kidney-inducible KO or kiKO). The urate/creatinine ratio was increased as early as 4 days after induction of the KO and no GLUT9 protein was visible on kidney extracts. kiKO mice are morphologically identical to their wild-type littermates and had no spontaneous kidney stones. Twenty-four-hour urine collection revealed a major increase of urate urinary excretion rate and of the fractional excretion of urate, with no difference in urate concentration in the plasma. Polyuria was observed, but kiKO mice were still able to concentrate urine after water restriction. KiKO mice displayed lower blood pressure accompanied by an increased heart rate. Overall, these results indicate that GLUT9 is a crucial player in renal handling of urate in vivo and a putative target for uricosuric drugs.
Keywords: GLUT9; Lithiasis; SLC2A9; Urate; Uric acid.
Figures
Similar articles
-
SGLT2 inhibition and renal urate excretion: role of luminal glucose, GLUT9, and URAT1.Am J Physiol Renal Physiol. 2019 Jan 1;316(1):F173-F185. doi: 10.1152/ajprenal.00462.2018. Epub 2018 Nov 14. Am J Physiol Renal Physiol. 2019. PMID: 30427222 Free PMC article.
-
Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia.Am J Hum Genet. 2008 Dec;83(6):744-51. doi: 10.1016/j.ajhg.2008.11.001. Epub 2008 Nov 20. Am J Hum Genet. 2008. PMID: 19026395 Free PMC article.
-
Glut9 is a major regulator of urate homeostasis and its genetic inactivation induces hyperuricosuria and urate nephropathy.Proc Natl Acad Sci U S A. 2009 Sep 8;106(36):15501-6. doi: 10.1073/pnas.0904411106. Epub 2009 Aug 21. Proc Natl Acad Sci U S A. 2009. PMID: 19706426 Free PMC article.
-
Urate Transporters in the Kidney: What Clinicians Need to Know.Electrolyte Blood Press. 2021 Jun;19(1):1-9. doi: 10.5049/EBP.2021.19.1.1. Epub 2021 Jun 30. Electrolyte Blood Press. 2021. PMID: 34290818 Free PMC article. Review.
-
Uric acid transport and disease.J Clin Invest. 2010 Jun;120(6):1791-9. doi: 10.1172/JCI42344. Epub 2010 Jun 1. J Clin Invest. 2010. PMID: 20516647 Free PMC article. Review.
Cited by
-
Age-dependent appearance of SARS-CoV-2 entry sites in mouse chemosensory systems reflects COVID-19 anosmia-ageusia symptoms.Commun Biol. 2021 Jul 15;4(1):880. doi: 10.1038/s42003-021-02410-9. Commun Biol. 2021. PMID: 34267318 Free PMC article.
-
Unraveling the impact of miRNAs on gouty arthritis: diagnostic significance and therapeutic opportunities.Naunyn Schmiedebergs Arch Pharmacol. 2025 Apr;398(4):3433-3450. doi: 10.1007/s00210-024-03603-9. Epub 2024 Nov 19. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39560752 Free PMC article. Review.
-
Kidney toxicology of a novel compound Lithium Bis(trifluoromethanesulfonyl)imide (LiTFSI, ie. HQ-115) used in energy applications: An epigenetic perspective.Sci Total Environ. 2024 Dec 10;955:177019. doi: 10.1016/j.scitotenv.2024.177019. Epub 2024 Oct 22. Sci Total Environ. 2024. PMID: 39447891 Free PMC article.
-
Chicken serum uric acid level is regulated by glucose transporter 9.Anim Biosci. 2021 Apr;34(4):670-679. doi: 10.5713/ajas.20.0092. Epub 2020 Jun 24. Anim Biosci. 2021. PMID: 32810934 Free PMC article.
-
The effects of GLUT9 and URAT1 inhibitors on cardiovascular diseases: a drug-targeted Mendelian randomization study.Int Urol Nephrol. 2025 Jun 6. doi: 10.1007/s11255-025-04594-z. Online ahead of print. Int Urol Nephrol. 2025. PMID: 40478372
References
-
- Anzai N, Ichida K, Jutabha P, Kimura T, Babu E, Jin CJ, Srivastava S, Kitamura K, Hisatome I, Endou H, Sakurai H. Plasma urate level is directly regulated by a voltage-driven urate efflux transporter URATv1 (SLC2A9) in humans. J Biol Chem. 2008;283:26834–26838. doi: 10.1074/jbc.C800156200. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

