Anti-Anginal Effectiveness and Tolerability of Trimetazidine Modified Release 80 Mg Once Daily in Stable Angina Patients in Real-World Practice
- PMID: 30105656
- PMCID: PMC6133142
- DOI: 10.1007/s12325-018-0756-3
Anti-Anginal Effectiveness and Tolerability of Trimetazidine Modified Release 80 Mg Once Daily in Stable Angina Patients in Real-World Practice
Abstract
Introduction: Trimetazidine (TMZ) was shown to reduce angina symptoms and increase the exercise capacity in stable angina (SA) patients. A new formulation allowing a once-daily (od) dosage could improve patients' satisfaction and adherence.
Methods: ODA was a 3-month, observational, multicenter, prospective Russian study in SA patients with persistent symptoms despite therapy. Angina attack frequency, short-acting nitrate (SAN) consumption, adherence to antianginal medications, and overall efficacy and tolerability of TMZ 80 mg od were assessed in a real-world setting.
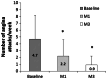
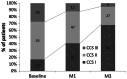
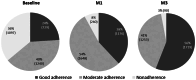
Results: A total of 3066 patients were included (mean age 62.8, 48% male). After 3 months, TMZ 80 mg od treatment led to a significant (p < 0.001) decrease in angina attack frequency (from 4.7 ± 3.5 to 0.9 ± 1.3/week) and SAN use (from 4.5 ± 3.9 to 0.7 ± 1.3/week). Overall tolerability and effectiveness were rated as "very good" by the majority of physicians. Medication adherence improved significantly, with good adherence reported by 56% of patients (vs. 24% at baseline, p < 0.0001) and non-adherence by 3% (vs. 36% at baseline, p < 0.0001) at month 3. Patient satisfaction with TMZ od was 9.5 [on a scale of 1 to 10 (very satisfied)]. Patients reported improved physical activity: more patients reported no limitations (15% vs. 1% at baseline p < 0.01), slight limitation (46% vs. 5% at baseline, p < 0.001) or moderate limitation (30% vs. 23%, p < 0.01) and fewer patients reported substantial limitation (8% vs. 52% at baseline, p < 0.001) or very marked reduction (1% vs. 19% at baseline, p < 0.01) at month 3.
Conclusion: In this prospective, observational study, TMZ 80 mg od effectively reduced angina attacks and SAN consumption, improved physical activity and adherence and was well tolerated in chronic SA patients.
Trial registration: ISRCTN registry Identifier, ISRCTN97780949.
Funding: Servier. Plain language summary available for this article.
Keywords: Cardiology; Observational study; Real-world evidence; Stable angina; Trimetazidine.
Conflict of interest statement
Maria G. Glezer, scientific coordinator of this study, received honoraria for lectures from Servier, Moscow, Russian Federation. Vladimir A. Vygodin has nothing to disclose.
Figures


References
-
- Montalescot G, Sechtem U, Achenbach S, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. 10.1093/eurheartj/eht310.P4876 - DOI - PubMed
-
- Beltrame JF, Weekes AJ, Morgan C, Tavella R, Spertus JA. The prevalence of weekly angina among patients with chronic stable angina in primary care practices: The Coronary Artery Disease in General Practice (CADENCE) Study. Arch Intern Med. 2009;169:1491–9. 10.1001/archinternmed.2009.295 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

