Proteomics: Clinical and research applications in respiratory diseases
- PMID: 30105802
- PMCID: PMC6234509
- DOI: 10.1111/resp.13383
Proteomics: Clinical and research applications in respiratory diseases
Abstract
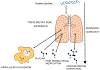
The proteome is the study of the protein content of a definable component of an organism in biology. However, the tissue-specific expression of proteins and the varied post-translational modifications, splice variants and protein-protein complexes that may form, make the study of protein a challenging yet vital tool in answering many of the unanswered questions in medicine and biology to date. Indeed, the spatial, temporal and functional composition of proteins in the human body has proven difficult to elucidate for many years. Given the effect of microRNA and epigenetic regulation on silencing and enhancing gene transcription, the study of protein arguably provides more accurate information on homeostasis and perturbation in health and disease. There have been significant advances in the field of proteomics in recent years, with new technologies and platforms available to the research community. In this review, we briefly discuss some of these new technologies and developments in the context of respiratory disease. We also discuss the types of data science approaches to analyses and interpretation of the large volumes of data generated in proteomic studies. We discuss the application of these technologies with regard to respiratory disease and highlight the potential for proteomics in generating major advances in the understanding of respiratory pathophysiology into the future.
Keywords: application; clinical; lung disease; proteomics; research.
© 2018 Asian Pacific Society of Respirology.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

