PGC-1α in hepatic UPR during high-fat high-fructose diet and exercise training in mice
- PMID: 30105901
- PMCID: PMC6090221
- DOI: 10.14814/phy2.13819
PGC-1α in hepatic UPR during high-fat high-fructose diet and exercise training in mice
Abstract
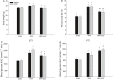
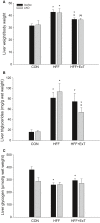
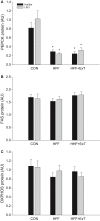
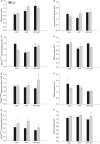
Diet-induced obesity is associated with hepatic steatosis, which has been linked with activation of the unfolded protein response (UPR). PGC-1α is a transcriptional coactivator involved in exercise training-induced adaptations in muscle and liver. Therefore, the aim of this study was to test the hypothesis that PGC-1α is required for exercise training-mediated prevention of diet-induced steatosis and UPR activation in liver. Male liver-specific PGC-1α knockout (LKO) and littermate floxed (lox/lox) mice were divided into two groups receiving either control diet (CON) or high-fat high-fructose diet (HFF). After 9 weeks, half of the HFF mice were treadmill exercise trained for 4 weeks (HFF+ExT), while the rest were kept sedentary. HFF resulted in increased body and liver weight, adiposity, hepatic steatosis and whole body glucose intolerance as well as decreased hepatic IRE1α phosphorylation. Exercise training prevented the HFF-induced weight gain and partially prevented increased liver weight, adiposity and glucose intolerance, but with no effect on liver triglycerides. In addition, BiP protein and CHOP mRNA content increased with exercise training compared with CON and HFF, respectively. Lack of PGC-1α in the liver only resulted in minor changes in the PERK pathway. In conclusion, this study provides evidence for dissociation between diet-induced hepatic triglyceride accumulation and hepatic UPR activation. In addition, PGC-1α was not required for maintenance of basal UPR in the liver and due to only minor exercise training effects on UPR further studies are needed to conclude on the potential role of PGC-1α in exercise training-induced adaptations in hepatic UPR.
Keywords: UPR; Exercise training; PGC-1α; high-fat diet; liver.
© 2018 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Figures

Similar articles
-
Impact of liver PGC-1α on exercise and exercise training-induced regulation of hepatic autophagy and mitophagy in mice on HFF.Physiol Rep. 2018 Jul;6(13):e13731. doi: 10.14814/phy2.13731. Physiol Rep. 2018. PMID: 29962089 Free PMC article.
-
PGC-1α in exercise and fasting-induced regulation of hepatic UPR in mice.Pflugers Arch. 2018 Oct;470(10):1431-1447. doi: 10.1007/s00424-018-2159-3. Epub 2018 Jun 30. Pflugers Arch. 2018. PMID: 29961149 Free PMC article.
-
PGC-1α in aging and lifelong exercise training-mediated regulation of UPR in mouse liver.Exp Gerontol. 2017 Nov;98:124-133. doi: 10.1016/j.exger.2017.08.006. Epub 2017 Aug 8. Exp Gerontol. 2017. PMID: 28801170
-
Autophagy activation, not peroxisome proliferator-activated receptor γ coactivator 1α, may mediate exercise-induced improvements in glucose handling during diet-induced obesity.Exp Physiol. 2017 Sep 1;102(9):1194-1207. doi: 10.1113/EP086406. Epub 2017 Jul 22. Exp Physiol. 2017. PMID: 28639297
-
Exercise training attenuates hepatic inflammation, fibrosis and macrophage infiltration during diet induced-obesity in mice.Brain Behav Immun. 2012 Aug;26(6):931-41. doi: 10.1016/j.bbi.2012.04.006. Epub 2012 Apr 23. Brain Behav Immun. 2012. PMID: 22554494
Cited by
-
Loss of TRIM67 Attenuates the Progress of Obesity-Induced Non-Alcoholic Fatty Liver Disease.Int J Mol Sci. 2022 Jul 5;23(13):7475. doi: 10.3390/ijms23137475. Int J Mol Sci. 2022. PMID: 35806477 Free PMC article.
-
Molecular cloning and characterization of endoplasmic reticulum stress related genes grp78 and atf6α from black seabream (Acanthopagrus schlegelii) and their expressions in response to nutritional regulation.Fish Physiol Biochem. 2023 Dec;49(6):1115-1128. doi: 10.1007/s10695-023-01242-0. Epub 2023 Oct 19. Fish Physiol Biochem. 2023. PMID: 37855969
-
Cross talk of vasopressin conditioned cell therapy in ischemic heart disease: Role of oxidative stress markers.Iran J Basic Med Sci. 2022 Sep;25(9):1084-1090. doi: 10.22038/IJBMS.2022.62540.13837. Iran J Basic Med Sci. 2022. PMID: 36246071 Free PMC article.
-
Aerobic Exercise-Assisted Cardiac Regeneration by Inhibiting Tryptase Release in Mast Cells after Myocardial Infarction.Biomed Res Int. 2021 Jun 8;2021:5521564. doi: 10.1155/2021/5521564. eCollection 2021. Biomed Res Int. 2021. PMID: 34212030 Free PMC article.
-
GDF15 is an exercise-induced hepatokine regulated by glucagon and insulin in humans.Front Endocrinol (Lausanne). 2022 Dec 5;13:1037948. doi: 10.3389/fendo.2022.1037948. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36545337 Free PMC article.
References
-
- Bertolotti, A. , Zhang Y., Hendershot L. M., Harding H. P., and Ron D.. 2000. Dynamic interaction of BiP and ER stress transducers in the unfolded‐protein response. Nat. Cell Biol. 2:326–332. - PubMed
-
- Chapados, N. A. , and Lavoie J. M.. 2010. Exercise training increases hepatic endoplasmic reticulum (er) stress protein expression in MTP‐inhibited high‐fat fed rats. Cell Biochem. Funct. 28:202–210. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

