Streptococcus pneumoniae Sialidase SpNanB-Catalyzed One-Pot Multienzyme (OPME) Synthesis of 2,7-Anhydro-Sialic Acids as Selective Sialidase Inhibitors
- PMID: 30105908
- PMCID: PMC6327951
- DOI: 10.1021/acs.joc.8b01519
Streptococcus pneumoniae Sialidase SpNanB-Catalyzed One-Pot Multienzyme (OPME) Synthesis of 2,7-Anhydro-Sialic Acids as Selective Sialidase Inhibitors
Abstract
Streptococcus pneumoniae sialidase SpNanB is an intramolecular trans-sialidase (IT-sialidase) and a virulence factor that is essential for streptococcal infection of the upper and lower respiratory tract. SpNanB catalyzes the formation of 2,7-anhydro- N-acetylneuraminic acid (2,7-anhydro-Neu5Ac), a potential prebiotic that can be used as the sole carbon source of a common human gut commensal anaerobic bacterium. We report here the development of an efficient one-pot multienzyme (OPME) system for synthesizing 2,7-anhydro-Neu5Ac and its derivatives. Based on a crystal structure analysis, an N-cyclohexyl derivative of 2,7-anhydro-neuraminic acid was designed, synthesized, and shown to be a selective inhibitor against SpNanB and another Streptococcus pneumoniae sialidase SpNanC. This study demonstrates a new strategy of synthesizing 2,7-anhydro-sialic acids in a gram scale and the potential application of their derivatives as selective sialidase inhibitors.
Conflict of interest statement
Notes
The authors declare no competing financial interest.
Figures
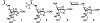



Similar articles
-
Structural and functional studies of Streptococcus pneumoniae neuraminidase B: An intramolecular trans-sialidase.FEBS Lett. 2008 Oct 15;582(23-24):3348-52. doi: 10.1016/j.febslet.2008.08.026. Epub 2008 Sep 5. FEBS Lett. 2008. PMID: 18775704
-
Membrane-enclosed multienzyme (MEME) synthesis of 2,7-anhydro-sialic acid derivatives.Carbohydr Res. 2017 Nov 8;451:110-117. doi: 10.1016/j.carres.2017.08.008. Epub 2017 Aug 19. Carbohydr Res. 2017. PMID: 28851488 Free PMC article.
-
The synthesis of a series of deoxygenated 2,3-difluoro-N-acteylneuraminic acid derivatives as potential sialidase inhibitors.Carbohydr Res. 2013 Jun 7;374:23-8. doi: 10.1016/j.carres.2013.03.026. Epub 2013 Apr 2. Carbohydr Res. 2013. PMID: 23618621
-
Sialidases from gut bacteria: a mini-review.Biochem Soc Trans. 2016 Feb;44(1):166-75. doi: 10.1042/BST20150226. Biochem Soc Trans. 2016. PMID: 26862202 Free PMC article. Review.
-
Diagnostics and Therapy of Human Diseases - Focus on Sialidases.Curr Pharm Des. 2018;24(24):2870-2875. doi: 10.2174/1381612824666180910125051. Curr Pharm Des. 2018. PMID: 30198428 Review.
Cited by
-
Elucidation of a sialic acid metabolism pathway in mucus-foraging Ruminococcus gnavus unravels mechanisms of bacterial adaptation to the gut.Nat Microbiol. 2019 Dec;4(12):2393-2404. doi: 10.1038/s41564-019-0590-7. Epub 2019 Oct 21. Nat Microbiol. 2019. PMID: 31636419 Free PMC article.
-
The putative Escherichia coli dehydrogenase YjhC metabolises two dehydrated forms of N-acetylneuraminate produced by some sialidases.Biosci Rep. 2020 Jun 26;40(6):BSR20200927. doi: 10.1042/BSR20200927. Biosci Rep. 2020. PMID: 32542330 Free PMC article.
-
Strategies for chemoenzymatic synthesis of carbohydrates.Carbohydr Res. 2019 Jan 15;472:86-97. doi: 10.1016/j.carres.2018.11.014. Epub 2018 Nov 24. Carbohydr Res. 2019. PMID: 30529493 Free PMC article. Review.
-
Sialidase Inhibitors with Different Mechanisms.J Med Chem. 2022 Oct 27;65(20):13574-13593. doi: 10.1021/acs.jmedchem.2c01258. Epub 2022 Oct 17. J Med Chem. 2022. PMID: 36252951 Free PMC article. Review.
-
Recent progress in chemical approaches for the development of novel neuraminidase inhibitors.RSC Adv. 2021 Jan 6;11(3):1804-1840. doi: 10.1039/d0ra07283d. eCollection 2021 Jan 4. RSC Adv. 2021. PMID: 35424082 Free PMC article. Review.
References
-
- Fine MJ; Smith MA; Carson CA; Mutha SS; Sankey SS; Weissfeld LA; Kapoor WN Prognosis And Outcomes of Patients with Community-Acquired Pneumonia: A Meta-Analysis. JAMA 1996, 275, 134–141. - PubMed
-
- Reichler MR; Allphin AA; Breiman RF; Schreiber JR; Arnold JE; McDougal LK; Facklam RR; Boxerbaum B; May D; Walton RO The Spread of Multiply Resistant Streptococcus pneumoniae at a Day Care Center in Ohio. J. Infect. Dis. 1992, 166, 1346–1353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

