Molecular Detection of Residual Parasitemia after Pyronaridine-Artesunate or Artemether-Lumefantrine Treatment of Uncomplicated Plasmodium falciparum Malaria in Kenyan Children
- PMID: 30105967
- PMCID: PMC6159604
- DOI: 10.4269/ajtmh.18-0233
Molecular Detection of Residual Parasitemia after Pyronaridine-Artesunate or Artemether-Lumefantrine Treatment of Uncomplicated Plasmodium falciparum Malaria in Kenyan Children
Abstract
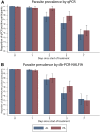
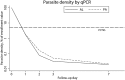
Artemisinin resistance is rapidly rising in Southeast Asia and may spread to African countries, where efficacy estimates are currently still excellent. Extensive monitoring of parasite clearance dynamics after treatment is needed to determine whether responsiveness to artemisinin-based combination therapies (ACT) is changing in Africa. In this study, Kenyan children with uncomplicated falciparum malaria were randomly assigned to pyronaridine-artesunate (PA) or artemether-lumefantrine (AL) treatment. Parasite clearance was evaluated over 7 days following the start of treatment by quantitative polymerase chain reaction (qPCR) and direct-on-blood PCR nucleic acid lateral flow immunoassay (db-PCR-NALFIA), a simplified molecular malaria diagnostic. Residual parasitemia at day 7 was detected by qPCR in 37.1% (26/70) of AL-treated children and in 46.1% (35/76) of PA-treated participants (P = 0.275). Direct-on-blood PCR nucleic acid lateral flow immunoassay detected residual parasites at day 7 in 33.3% (23/69) and 30.3% (23/76) of AL and PA-treated participants, respectively (P = 0.692). qPCR-determined parasitemia at day 7 was associated with increased prevalence and density of gametocytes at baseline (P = 0.014 and P = 0.003, for prevalence and density, respectively) and during follow-up (P = 0.007 and P = 0.011, respectively, at day 7). A positive db-PCR-NALFIA outcome at day 7 was associated with treatment failure (odds ratio [OR]: 3.410, 95% confidence interval [CI]: 1.513-7.689, P = 0.003), but this association was not found for qPCR (OR: 0.701, 95% CI: 0.312-1.578, P = 0.391). Both qPCR and db-PCR-NALFIA detected substantial residual submicroscopic parasitemia after microscopically successful PA and AL treatment and can be useful tools to monitor parasite clearance. To predict treatment outcome, db-PCR-NALFIA may be more suitable than qPCR.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

