Scutellarin protects oxygen/glucose-deprived astrocytes and reduces focal cerebral ischemic injury
- PMID: 30106052
- PMCID: PMC6108207
- DOI: 10.4103/1673-5374.235293
Scutellarin protects oxygen/glucose-deprived astrocytes and reduces focal cerebral ischemic injury
Abstract
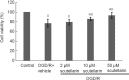
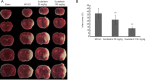
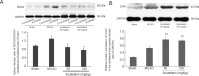
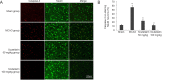
Scutellarin, a bioactive flavone isolated from Scutellaria baicalensis, has anti-inflammatory, anti-neurotoxic, anti-apoptotic and anti-oxidative effects and has been used to treat cardiovascular and cerebrovascular diseases in China. However, the mechanisms by which scutellarin mediates neuroprotection in cerebral ischemia remain unclear. The interaction between scutellarin and nicotinamide adenine dinucleotide phosphate oxidase 2 (NOX2) was assessed by molecular docking study, which showed that scutellarin selectively binds to NOX2 with high affinity. Cultures of primary astrocytes isolated from the cerebral cortex of neonatal Sprague-Dawley rats were pretreated with 2, 10 or 50 μM scutellarin for 30 minutes. The astrocytes were then subjected to oxygen/glucose deprivation by incubation for 2 hours in glucose-free Dulbecco's modified Eagle's medium in a 95% N2/5% CO2 incubator, followed by simulated reperfusion for 22 hours. Cell viability was assessed by cell counting kit-8 assay. Expression levels of NOX2, connexin 43 and caspase-3 were assessed by western blot assay. Reactive oxygen species were measured spectrophotometrically. Pretreatment with 10 or 50 μM scutellarin substantially increased viability, reduced the expression of NOX2 and caspase-3, increased the expression of connexin 43, and diminished the levels of reactive oxygen species in astrocytes subjected to ischemia-reperfusion. We also assessed the effects of scutellarin in vivo in the rat transient middle cerebral artery occlusion model of cerebral ischemia-reperfusion injury. Rats were given intraperitoneal injection of 100 mg/kg scutellarin 2 hours before surgery. The Bederson scale was used to assess neurological deficit, and 2,3,5-triphenyltetrazolium chloride staining was used to measure infarct size. Western blot assay was used to assess expression of NOX2 and connexin 43 in brain tissue. Enzyme-linked immunosorbent assay was used to detect 8-hydroxydeoxyguanosine (8-OHdG), 4-hydroxy-2-nonenal (4-HNE) and 3-nitrotyrosin (3-NT) in brain tissue. Immunofluorescence double staining was used to determine the co-expression of caspase-3 and NeuN. Pretreatment with scutellarin improved the neurological function of rats with focal cerebral ischemia, reduced infarct size, diminished the expression of NOX2, reduced levels of 8-OHdG, 4-HNE and 3-NT, and reduced the number of cells co-expressing caspase-3 and NeuN in the injured brain tissue. Furthermore, we examined the effect of the NOX2 inhibitor apocynin. Apocynin substantially increased connexin 43 expression in vivo and in vitro. Collectively, our findings suggest that scutellarin protects against ischemic injury in vitro and in vivo by downregulating NOX2, upregulating connexin 43, decreasing oxidative damage, and reducing apoptotic cell death.
Keywords: cerebral ischemic injury; connexin 43; nerve regeneration; neural regeneration; nicotinamide adenine dinucleotide phosphate oxidase 2; oxygen glucose deprivation/reoxygenation; reactive oxygen species; scutellarin.
Conflict of interest statement
None declared
Figures




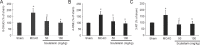

References
-
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

