Novel conductive polypyrrole/silk fibroin scaffold for neural tissue repair
- PMID: 30106059
- PMCID: PMC6108196
- DOI: 10.4103/1673-5374.235303
Novel conductive polypyrrole/silk fibroin scaffold for neural tissue repair
Abstract
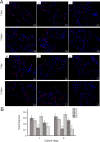
Three dimensional (3D) bioprinting, which involves depositing bioinks (mixed biomaterials) layer by layer to form computer-aided designs, is an ideal method for fabricating complex 3D biological structures. However, it remains challenging to prepare biomaterials with micro-nanostructures that accurately mimic the nanostructural features of natural tissues. A novel nanotechnological tool, electrospinning, permits the processing and modification of proper nanoscale biomaterials to enhance neural cell adhesion, migration, proliferation, differentiation, and subsequent nerve regeneration. The composite scaffold was prepared by combining 3D bioprinting with subsequent electrochemical deposition of polypyrrole and electrospinning of silk fibroin to form a composite polypyrrole/silk fibroin scaffold. Fourier transform infrared spectroscopy was used to analyze scaffold composition. The surface morphology of the scaffold was observed by light microscopy and scanning electron microscopy. A digital multimeter was used to measure the resistivity of prepared scaffolds. Light microscopy was applied to observe the surface morphology of scaffolds immersed in water or Dulbecco's Modified Eagle's Medium at 37°C for 30 days to assess stability. Results showed characteristic peaks of polypyrrole and silk fibroin in the synthesized conductive polypyrrole/silk fibroin scaffold, as well as the structure of the electrospun nanofiber layer on the surface. The electrical conductivity was 1 × 10-5-1 × 10-3 S/cm, while stability was 66.67%. A 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide assay was employed to measure scaffold cytotoxicity in vitro. Fluorescence microscopy was used to observe EdU-labeled Schwann cells to quantify cell proliferation. Immunohistochemistry was utilized to detect S100β immunoreactivity, while scanning electron microscopy was applied to observe the morphology of adherent Schwann cells. Results demonstrated that the polypyrrole/silk fibroin scaffold was not cytotoxic and did not affect Schwann cell proliferation. Moreover, filopodia formed on the scaffold and Schwann cells were regularly arranged. Our findings verified that the composite polypyrrole/silk fibroin scaffold has good biocompatibility and may be a suitable material for neural tissue engineering.
Keywords: L929 cells; Schwann cells; biocompatibility; composite nanofiber; conductivity; electrospinning; nerve regeneration; nerve repair; neural regeneration; polypyrrole; scaffold; silk fibroin; three dimensional bioprinting.
Conflict of interest statement
There are no conflicts of interest to declare
Figures





References
-
- Aznar-Cervantes S, Roca MI, Martinez JG, Meseguer-Olmo L, Cenis JL, Moraleda JM, Otero TF. Fabrication of conductive electrospun silk fibroin scaffolds by coating with polypyrrole for biomedical applications. Bioelectrochemistry. 2012;85:36–43. - PubMed
-
- Baniasadi H, Ramazani SAA, Mashayekhan S. Fabrication and characterization of conductive chitosan/gelatin-based scaffolds for nerve tissue engineering. Int J Biol Macromol. 2015;74:360–366. - PubMed
-
- Belavi PB, Chavan GN, Naik LR, Somashekar R, Kotnala RK. Structural electrical and magnetic properties of cadmium substituted nickel–copper ferrites. Mater Chem Phys. 2012;132:138–144.
LinkOut - more resources
Full Text Sources
Other Literature Sources

