Silencing of PYGB suppresses growth and promotes the apoptosis of prostate cancer cells via the NF‑κB/Nrf2 signaling pathway
- PMID: 30106110
- PMCID: PMC6131497
- DOI: 10.3892/mmr.2018.9388
Silencing of PYGB suppresses growth and promotes the apoptosis of prostate cancer cells via the NF‑κB/Nrf2 signaling pathway
Abstract
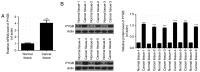
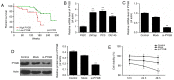
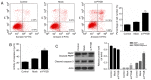
Brain‑type glycogen phosphorylase (PYGB) is an enzyme that metabolizes glycogen, whose function is to provide energy for an organism in an emergency state. The present study purposed to investigate the role and mechanism of PYGB silencing on the growth and apoptosis of prostate cancer cells. A cell counting kit‑8 assay and flow cytometry were performed to determine the cell viability, apoptosis and reactive oxygen species (ROS) content, respectively. Colorimetry was performed to analyze the activity of caspase‑3. Western blotting and reverse transcription‑quantitative polymerase chain reaction were used to evaluate the associated mRNA and protein expression levels. The results revealed that PYGB was upregulated in prostate cancer tissues and was associated with disease progression. In addition, PYGB silencing suppressed the cell viability of PC3 cells. PYGB silencing promoted apoptosis of PC3 cells via the regulation of the expression levels of cleaved‑poly (adenosine diphosphate‑ribose) polymerase, cleaved‑caspase‑3, B‑cell lymphoma‑2 (Bcl‑2) and Bcl‑2‑associated X protein. PYGB silencing increased the ROS content in PC3 cells, and affected nuclear factor (NF)‑κB/nuclear factor‑erythroid 2‑related factor 2 (Nrf2) signaling pathways in PC3 cells. In conclusion, PYGB silencing suppressed the growth and promoted the apoptosis of prostate cancer cells by affecting the NF‑κB/Nrf2 signaling pathway. The present study provided evidence that may lead to the development of a potential therapeutic strategy for prostate cancer.
Figures



References
-
- Cai C, Chen S, Ng P, Bubley GJ, Nelson PS, Mostaghel EA, Marck B, Matsumoto AM, Simon NI, Wang H, et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res. 2011;71:6503–6513. doi: 10.1158/1538-7445.AM2011-LB-420. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

