A natural ent‑kaurane diterpenoid induces antiproliferation in ovarian cancer cells via ERK1/2 regulation and inhibition of cellular migration and invasion
- PMID: 30106144
- PMCID: PMC6131655
- DOI: 10.3892/mmr.2018.9377
A natural ent‑kaurane diterpenoid induces antiproliferation in ovarian cancer cells via ERK1/2 regulation and inhibition of cellular migration and invasion
Abstract
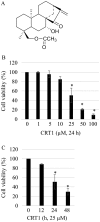
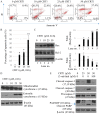
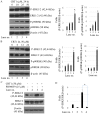
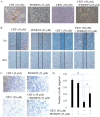
Ovarian cancer is one of the most common causes of female mortalities from gynecological tumors. An ent‑kaurane diterpenoid compound CRT1 (ent‑18‑acetoxy‑7β‑hydroxy kaur‑15‑oxo‑16‑ene), mainly isolated from the Vietnamese herb Croton tonkinesis has been used in folk medicine in Vietnam for cancer treatment. However, the effect of this compound on human ovarian cancer cells has not yet been reported. The objective of the present study was to determine the effect of CRT1 on the cell viability, apoptosis and metastasis of SKOV3 human ovarian cancer cells using a Cell Counting Kit‑8 assay, flow cytometric analysis of Annexin V‑fluorescein isothiocyanate/propidium iodide staining, western blot analysis, soft agar colony forming assay, wound healing assay and Matrigel invasion assay. The results revealed that CRT1 possessed significant anti‑proliferative effects on SKOV3 cells. CRT1 treatment at 25 and 50 µM induced apoptosis, enhanced the percentage of Annexin V‑positive cells, increased the expression of pro‑apoptotic protein B‑cell lymphoma 2 (Bcl‑2)‑associated X protein, cytochrome c release from the mitochondria to the cytosol, cleaved caspase‑3, caspase‑7, caspase‑9, and poly (adenosine diphosphate‑ribose) polymerase. However, it decreased the expression of Bcl‑2 in a dose‑dependent manner. The percentage of necrotic cells increased following CRT1 treatment at <10 µM. CRT1 at 50 µM significantly induced the phosphorylation of extracellular signal‑regulated kinase (ERK). Growth inhibition and the apoptotic effects of CRT1 could be reversed by PD98059, an ERK inhibitor. Additionally, CRT1 inhibited cell migration and invasion via ERK1/2 activation in SKOV3 cells. These results indicated that CRT1, an ent‑kaurane diterpenoid, may be a potential inhibitor of ovarian cancer by the activating ERK1/2/p90 ribosomal S6 kinase signaling pathway.
Figures

Similar articles
-
Alpinetin inhibits proliferation and migration of ovarian cancer cells via suppression of STAT3 signaling.Mol Med Rep. 2018 Oct;18(4):4030-4036. doi: 10.3892/mmr.2018.9420. Epub 2018 Aug 22. Mol Med Rep. 2018. PMID: 30132572
-
An ent-kaurane diterpenoid from Croton tonkinensis induces apoptosis by regulating AMP-activated protein kinase in SK-HEP1 human hepatocellular carcinoma cells.Biol Pharm Bull. 2013;36(1):158-64. doi: 10.1248/bpb.b12-00873. Biol Pharm Bull. 2013. PMID: 23302650
-
Honokiol induces apoptosis and suppresses migration and invasion of ovarian carcinoma cells via AMPK/mTOR signaling pathway.Int J Mol Med. 2019 May;43(5):1969-1978. doi: 10.3892/ijmm.2019.4122. Epub 2019 Mar 5. Int J Mol Med. 2019. PMID: 30864681 Free PMC article.
-
[Expression and significance of MAPK/ERK in the specimens and cells of epithelial ovarian cancer].Zhonghua Fu Chan Ke Za Zhi. 2019 Aug 25;54(8):541-547. doi: 10.3760/cma.j.issn.0529-567x.2019.08.007. Zhonghua Fu Chan Ke Za Zhi. 2019. PMID: 31461811 Chinese.
-
Oridonin Suppresses Proliferation of Human Ovarian Cancer Cells via Blockage of mTOR Signaling.Asian Pac J Cancer Prev. 2016;17(2):667-71. doi: 10.7314/apjcp.2016.17.2.667. Asian Pac J Cancer Prev. 2016. PMID: 26925661
Cited by
-
ERK: A Double-Edged Sword in Cancer. ERK-Dependent Apoptosis as a Potential Therapeutic Strategy for Cancer.Cells. 2021 Sep 22;10(10):2509. doi: 10.3390/cells10102509. Cells. 2021. PMID: 34685488 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

