PI3K/Akt and HIF‑1 signaling pathway in hypoxia‑ischemia (Review)
- PMID: 30106145
- PMCID: PMC6131612
- DOI: 10.3892/mmr.2018.9375
PI3K/Akt and HIF‑1 signaling pathway in hypoxia‑ischemia (Review)
Abstract
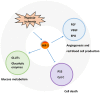
Hypoxia‑ischemia (H‑I) is frequently observed in perinatal asphyxia and other diseases. It can lead to serious cardiac injury, cerebral damage, neurological disability and mortality. Previous studies have demonstrated that the phosphatidylinositol‑3 kinase (PI3K)/protein kinase B (Akt) signaling pathway, which regulates a wide range of cellular functions, is involved in the resistance response to H‑I through the activation of proteins associated with survival and inactivation of apoptosis‑associated proteins. It can also regulate the expression of hypoxia‑induced factor‑1α (HIF‑1α). HIF‑1α can further regulate the expression of downstream proteins involved in glucose metabolism and angiogenesis, such as vascular endothelial growth factor and erythropoietin, to facilitate ischemic adaptation. Notably, HIF‑1α may also induce detrimental effects. The effects of HIF‑1 on ischemic outcomes may be dependent on the H‑I duration, animal age and species. Thus, further investigation of the PI3K/Akt signaling pathway may provide further insights of the potential targets for treating diseases accompanied by H‑I.
Figures

References
-
- Adluri RS, Thirunavukkarasu M, Zhan L, Akita Y, Samuel SM, Otani H, Ho YS, Maulik G, Maulik N. Thioredoxin 1 enhances neovascularization and reduces ventricular remodeling during chronic myocardial infarction: A study using thioredoxin 1 transgenic mice. J Mol Cell Cardiol. 2011;50:239–247. doi: 10.1016/j.yjmcc.2010.11.002. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

