D-cycloserine-augmented one-session treatment of specific phobias in children and adolescents
- PMID: 30106248
- PMCID: PMC5991588
- DOI: 10.1002/brb3.984
D-cycloserine-augmented one-session treatment of specific phobias in children and adolescents
Abstract
Background: D-Cycloserine has potential to enhance exposure therapy outcomes. The current study presents a preliminary randomized, placebo-controlled double-blind pilot trial of DCS-augmented one-session treatment (OST) for youth (7-14 years) with specific phobia. A secondary aim of this pilot study was to explore the effects of youth age and within-session fear reduction as potential moderators of DCS outcomes in order to generate hypotheses for a larger trial. It was hypothesized that DCS would be associated with greater improvements than placebo, that children (7-10 years) would have greater benefits than adolescents (11-14 years), and that DCS effects would be stronger for participants with the greater within-session fear reduction during the OST.
Methods: Thirty-five children and adolescents were randomized to either OST combined with DCS (n = 17), or OST combined with placebo (PBO; n = 18) and assessed at 1 week, 1 month, and 3 month following treatment.
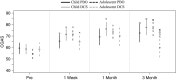
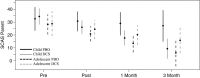
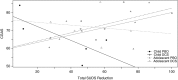
Results: There were no significant pre- to post-treatment or follow-up benefits of DCS relative to placebo. Secondary analyses of age indicated that relative to PBO, DCS was associated with greater improvements for children (but not adolescents) on measures of severity at 1-month follow-up. Children in the DCS condition also showed significantly greater improvement to 1 month on global functioning relative to other groups. Conversely, adolescents had significant post-treatment benefits in the PBO condition on symptom severity measures relative to DCS, and adolescents in the DCS condition had significantly poorer functioning at 3 months relative to all other groups. Finally, there was a trend for within-session fear reduction to be associated with moderating effects of DCS, whereby greater reduction in fear was associated with greater functioning at one-month follow-up for children who received DCS, relative to PBO.
Limitations: The study sample was small and therefore conclusions are tentative and require replication.
Conclusions: Age and within-session fear reduction may be important moderators of DCS-augmented one-session exposure therapy, which requires testing in a fully powered randomized controlled trial.
Keywords: D‐Cycloserine; children; exposure therapy; one‐session treatment; phobia.
© 2018 The Authors. Brain and Behavior published by Wiley Periodicals, Inc.
Figures


References
-
- Baker, K. D. , & Richardson, R. (2015). Forming competing fear learning and extinction memories in adolescence makes fear difficult to inhibit. Learning & Memory, 22, 537–543. https://doi.org/10.1101/lm.039487.114 - DOI - PMC - PubMed
-
- Bener, A. , Ghuloum, S. , & Dafeeah, E. E. (2011). Prevalence of common phobias and their socio‐demographic correlates in children and adolescents in a traditional developing society. African Journal of Psychiatry, 14, 140–145. - PubMed
-
- Byrne, S. P. , Farrell, L. J. , Storch, E. , & Rapee, R. M. (2014). D‐cycloserine augmented treatment of anxiety disorders in children and adolescents: a review of preliminary research. Psychopathology Review, 1(1), 157–168. https://doi.org/10.5127/pr.033013 - DOI
-
- Byrne, S. P. , Rapee, R. M. , Richardson, R. , Malhi, G. S. , Jones, M. , & Hudson, J. L. (2015). D‐cycloserine enhances generalization of fear extinction in children. Depress Anxiety, 32, 408–414. https://doi.org/10.1002/da.22356 - DOI - PubMed
-
- Davis, T. E. III , & Ollendick, T. H. (2005). Empirically supported treatments for specific phobia in children: do efficacious treatments address the components of a phobic response? Clinical Psychology: Science and Practice., 12, 144–160.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

