Functional gradients of the cerebellum
- PMID: 30106371
- PMCID: PMC6092123
- DOI: 10.7554/eLife.36652
Functional gradients of the cerebellum
Abstract
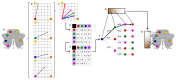
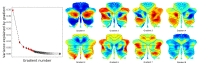
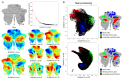
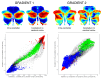
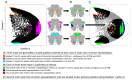
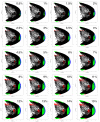
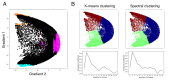
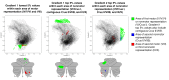
A central principle for understanding the cerebral cortex is that macroscale anatomy reflects a functional hierarchy from primary to transmodal processing. In contrast, the central axis of motor and nonmotor macroscale organization in the cerebellum remains unknown. Here we applied diffusion map embedding to resting-state data from the Human Connectome Project dataset (n = 1003), and show for the first time that cerebellar functional regions follow a gradual organization which progresses from primary (motor) to transmodal (DMN, task-unfocused) regions. A secondary axis extends from task-unfocused to task-focused processing. Further, these two principal gradients revealed novel functional properties of the well-established cerebellar double motor representation (lobules I-VI and VIII), and its relationship with the recently described triple nonmotor representation (lobules VI/Crus I, Crus II/VIIB, IX/X). Functional differences exist not only between the two motor but also between the three nonmotor representations, and second motor representation might share functional similarities with third nonmotor representation.
Keywords: cerebellum; fMRI; functional organization; functional topography; gradients; human; neuroscience; resting-state.
© 2018, Guell et al.
Conflict of interest statement
XG, JS, JG, SG No competing interests declared
Figures





References
-
- Arnold Anteraper S, Guell X, D'Mello A, Joshi N, Whitfield-Gabrieli S, Joshi G. Disrupted cerebrocerebellar intrinsic functional connectivity in young adults with High-Functioning autism spectrum disorder: a Data-Driven, Whole-Brain, High-Temporal resolution functional magnetic resonance imaging study. Brain Connectivity. 2018 doi: 10.1089/brain.2018.0581. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

