Stimulus vignetting and orientation selectivity in human visual cortex
- PMID: 30106372
- PMCID: PMC6092116
- DOI: 10.7554/eLife.37241
Stimulus vignetting and orientation selectivity in human visual cortex
Abstract
Neural selectivity to orientation is one of the simplest and most thoroughly-studied cortical sensory features. Here, we show that a large body of research that purported to measure orientation tuning may have in fact been inadvertently measuring sensitivity to second-order changes in luminance, a phenomenon we term 'vignetting'. Using a computational model of neural responses in primary visual cortex (V1), we demonstrate the impact of vignetting on simulated V1 responses. We then used the model to generate a set of predictions, which we confirmed with functional MRI experiments in human observers. Our results demonstrate that stimulus vignetting can wholly determine the orientation selectivity of responses in visual cortex measured at a macroscopic scale, and suggest a reinterpretation of a well-established literature on orientation processing in visual cortex.
Keywords: fMRI; human; multivariate classification; neuroscience; orientation; primary visual cortex.
Conflict of interest statement
ZR, DH, EM No competing interests declared
Figures
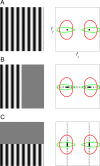





Comment in
-
Framing orientation selectivity.Elife. 2018 Aug 14;7:e39762. doi: 10.7554/eLife.39762. Elife. 2018. PMID: 30106374 Free PMC article.
Similar articles
-
Natural scene sampling reveals reliable coarse-scale orientation tuning in human V1.Nat Commun. 2022 Oct 29;13(1):6469. doi: 10.1038/s41467-022-34134-7. Nat Commun. 2022. PMID: 36309512 Free PMC article.
-
Edge-Related Activity Is Not Necessary to Explain Orientation Decoding in Human Visual Cortex.J Neurosci. 2017 Feb 1;37(5):1187-1196. doi: 10.1523/JNEUROSCI.2690-16.2016. Epub 2016 Dec 21. J Neurosci. 2017. PMID: 28003346 Free PMC article.
-
Sound Induces Change in Orientation Preference of V1 Neurons: Audio-Visual Cross-Influence.Neuroscience. 2019 Apr 15;404:48-61. doi: 10.1016/j.neuroscience.2019.01.039. Epub 2019 Jan 29. Neuroscience. 2019. PMID: 30703505
-
Surround suppression supports second-order feature encoding by macaque V1 and V2 neurons.Vision Res. 2014 Nov;104:24-35. doi: 10.1016/j.visres.2014.10.004. Epub 2014 Oct 23. Vision Res. 2014. PMID: 25449336 Free PMC article. Review.
-
Functional cell classes and functional architecture in the early visual system of a highly visual rodent.Prog Brain Res. 2005;149:127-45. doi: 10.1016/S0079-6123(05)49010-X. Prog Brain Res. 2005. PMID: 16226581 Review.
Cited by
-
Artificial Visual System for Orientation Detection Based on Hubel-Wiesel Model.Brain Sci. 2022 Apr 1;12(4):470. doi: 10.3390/brainsci12040470. Brain Sci. 2022. PMID: 35448001 Free PMC article.
-
Framing orientation selectivity.Elife. 2018 Aug 14;7:e39762. doi: 10.7554/eLife.39762. Elife. 2018. PMID: 30106374 Free PMC article.
-
Orientation anisotropies in macaque visual areas.Proc Natl Acad Sci U S A. 2022 Apr 12;119(15):e2113407119. doi: 10.1073/pnas.2113407119. Epub 2022 Apr 5. Proc Natl Acad Sci U S A. 2022. PMID: 35380895 Free PMC article.
-
Opposing effects of selectivity and invariance in peripheral vision.Nat Commun. 2021 Jul 28;12(1):4597. doi: 10.1038/s41467-021-24880-5. Nat Commun. 2021. PMID: 34321483 Free PMC article.
-
Testing cognitive theories with multivariate pattern analysis of neuroimaging data.Nat Hum Behav. 2023 Sep;7(9):1430-1441. doi: 10.1038/s41562-023-01680-z. Epub 2023 Aug 17. Nat Hum Behav. 2023. PMID: 37591984 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

