Prognostication of chronic disorders of consciousness using brain functional networks and clinical characteristics
- PMID: 30106378
- PMCID: PMC6145856
- DOI: 10.7554/eLife.36173
Prognostication of chronic disorders of consciousness using brain functional networks and clinical characteristics
Abstract
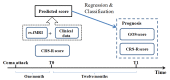
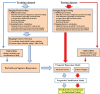
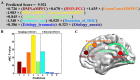
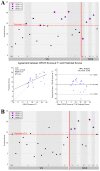
Disorders of consciousness are a heterogeneous mixture of different diseases or injuries. Although some indicators and models have been proposed for prognostication, any single method when used alone carries a high risk of false prediction. This study aimed to develop a multidomain prognostic model that combines resting state functional MRI with three clinical characteristics to predict one year-outcomes at the single-subject level. The model discriminated between patients who would later recover consciousness and those who would not with an accuracy of around 88% on three datasets from two medical centers. It was also able to identify the prognostic importance of different predictors, including brain functions and clinical characteristics. To our knowledge, this is the first reported implementation of a multidomain prognostic model that is based on resting state functional MRI and clinical characteristics in chronic disorders of consciousness, which we suggest is accurate, robust, and interpretable.
Keywords: brain; functional network; human; human biology; medicine; neural network; neuroscience.
© 2018, Song et al.
Conflict of interest statement
MS, YY, JH, ZY, SY, QX, XX, YD, QZ, XW, YC, BH, RY, RX, TJ No competing interests declared
Figures


















References
-
- Bernat JL. Prognostic limitations of syndromic diagnosis in disorders of consciousness. AJOB Neuroscience. 2016;7:46–48. doi: 10.1080/21507740.2016.1146367. - DOI
-
- Bruno M-A, Ledoux D, Vanhaudenhuyse A, Gosseries O, Thibaut A, Laureys S. Prognosis of Patients with Altered State of Consciousness. In: Schnakers C, Laureys S, editors. Coma and Disorders of Consciousness. London: Springer London. 2012. pp. 11–23. - DOI
Publication types
MeSH terms
Grants and funding
- 81471380/National Natural Science Foundation of China/International
- Z161100000216152/Beijing Municipal Science and Technology Commission/International
- Z161100000516165/Beijing Municipal Scienceand Technology Commission/International
- 2017YFA0105203/National Key R&D Program of China/International
- 31620103905/National Natural Science Foundation of China/International
- 91432302/National Natural Science Foundation of China/International
- Science Frontier Program: QYZDJ-SSW-SMC019/Chinese Academy of Sciences/International
- Z161100000216139/Beijing Municipal Science and Technology Commission/International
- 2016ZT06S220/Guangdong Pearl River Talents Plan Innovative and Entrepreneurial Team/International
- 91432302,31620103905/National Natural Science Foundation of China/International
- QYZDJ-SSW-SMC019/The Science Frontier Program of the Chinese academy of Sciences/International
- Z161100000516165/Beijing Municipal Science and Technology Commission/International
- 2016ZT06S220/The Guangdong Pearl River Talents Plan Innovative and Entrepreneurial Team/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

