Tumor Mutation Burden as a Biomarker in Resected Non-Small-Cell Lung Cancer
- PMID: 30106638
- PMCID: PMC6804865
- DOI: 10.1200/JCO.2018.78.1963
Tumor Mutation Burden as a Biomarker in Resected Non-Small-Cell Lung Cancer
Abstract
Purpose: The survival benefit with adjuvant chemotherapy for patients with resected stage II-III non-small-cell lung cancer (NSCLC) is modest. Efforts to develop prognostic or predictive biomarkers in these patients have not yielded clinically useful tests. We report findings from the Lung Adjuvant Cisplatin Evaluation (LACE)-Bio-II study, in which we analyzed next-generation sequencing and long-term outcomes data from > 900 patients with early-stage NSCLC treated prospectively in adjuvant landmark clinical trials. We used a targeted gene panel to assess the prognostic and predictive effect of mutations in individual genes, DNA repair pathways, and tumor mutation burden (TMB).
Methods: A total of 908 unmatched, formalin-fixed, paraffin-embedded, resected lung cancer tumor specimens were sequenced using a targeted panel of 1,538 genes. Stringent filtering criteria were applied to exclude germline variants and artifacts related to formalin fixation. Disease-free survival, overall survival, and lung cancer-specific survival (LCSS) were assessed in Cox models stratified by trial and adjusted for treatment, age, sex, performance score, histology, type of surgery, and stage.
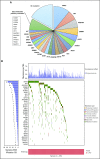
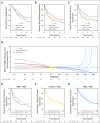
Results: Nonsynonymous mutations were identified in 1,515 genes in 908 tumor samples. High nonsynonymous TMB (> 8 mutations/Mb) was prognostic for favorable outcomes (ie, overall survival, disease-free survival, and LCSS) in patients with resected NSCLC. LCSS benefit with adjuvant chemotherapy was more pronounced in patients with low nonsynonymous TMBs (≤ 4 mutations/Mb). Presence of mutations in DNA repair pathways, tumor-infiltrating lymphocytes, TP53 alteration subtype, and intratumor heterogeneity was neither prognostic nor predictive. Statistically significant effect of mutations in individual genes was difficult to determine due to high false-discovery rates.
Conclusion: High nonsynonymous TMB was associated with a better prognosis in patients with resected NSCLC. In addition, the benefit of adjuvant chemotherapy on LCSS was more pronounced in patients with low nonsynonymous TMBs. Studies are warranted to confirm these findings.
Figures

Comment in
-
Tumor Mutation Burden: Is It Ready for the Clinic?J Clin Oncol. 2018 Oct 20;36(30):2978-2979. doi: 10.1200/JCO.2018.79.3398. Epub 2018 Sep 4. J Clin Oncol. 2018. PMID: 30179566 No abstract available.
References
-
- Pignon JP, Tribodet H, Scagliotti GV, et al. : Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE Collaborative Group. J Clin Oncol 26:3552-3559, 2008 - PubMed
-
- Douillard JY, Rosell R, De Lena M, et al. : Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): A randomised controlled trial. Lancet Oncol 7:719-727, 2006 - PubMed
-
- Strauss GM, Herndon JE, II, Maddaus MA, et al. : Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 26:5043-5051, 2008 - PMC - PubMed
-
- Arriagada R, Bergman B, Dunant A, et al. : Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 350:351-360, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

