Dexmedetomidine in combination with morphine improves postoperative analgesia and sleep quality in elderly patients after open abdominal surgery: A pilot randomized control trial
- PMID: 30106963
- PMCID: PMC6091958
- DOI: 10.1371/journal.pone.0202008
Dexmedetomidine in combination with morphine improves postoperative analgesia and sleep quality in elderly patients after open abdominal surgery: A pilot randomized control trial
Abstract
Background: Dexmedetomidine in combination with opioids has been used for postoperative analgesia. The purpose of this study was to investigate the impacts of dexmedetomidine supplemented intravenous analgesia on morphine consumption and subjective sleep quality in elderly patients after open abdominal surgery.
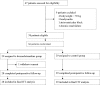
Methods: This was a pilot randomized controlled trial. 58 elderly patients (age ≥ 60 years) who underwent open abdominal surgery were randomized to receive either dexmedetomidine supplemented morphine analgesia (0.5 mg/ml morphine plus 2 μg/ml dexmedetomidine in 100 ml normal saline, DEX group) or morphine analgesia (0.5 mg/ml morphine in 100 ml normal saline, CTRL group) for 72 hours after surgery. Patient-controlled analgesia pump was programmed to deliver a 2ml bolus with a lockout interval of 8 minutes and a background infusion at a rate of 1 ml/h. The primary endpoint was 72-hour morphine consumption. Secondary endpoints included pain intensity, subjective sleep quality, and 30-day complications and mortality after surgery.
Results: The 72-hour morphine consumption was lower in the DEX group than in the CTRL group (median 39.0 mg [interquartile range 37.3, 41.0] in the DEX group vs. 49.0 mg [45.5, 50.0] in the CTRL group; median difference -9.0 mg [95% CI -10.0, -6.0], P < 0.001). The intensity of pain within 48 hours was lower (P<0.001 at 4, 12 and 48 hours, P = 0.007 at 24 hours) whereas the subjective quality of sleep was higher (P = 0.031 during the night of surgery and P<0.001 during the 1st night after surgery, respectively) in the DEX group than in the CTRL group. The incidence of 30-day complications did not differ significantly between groups, but it was slightly lower in the DEX group (P = 0.060). There were no significant differences between groups regarding 30-day mortality and the incidences of adverse events.
Conclusions: For elderly patients after open abdominal surgery, dexmedetomidine supplemented analgesia decreases morphine consumption, improves analgesic effects and subjective sleep quality without increasing adverse events.
Trial registration: Chinese Clinical Trial Registry ChiCTR-IPR-14005620.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003. August; 97(2): 534–40. - PubMed
-
- American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the peri-operative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology 2012; 116: 248–73 10.1097/ALN.0b013e31823c1030 - DOI - PubMed
-
- Langford RM, Joshi GP, Gan TJ, Mattera MS, Chen WH, Revicki DA, et al. Reduction in opioid-related adverse events and improvement in function with parecoxib followed by valdecoxib treatment after non-cardiac surgery: a randomized, double-blind, placebo-controlled, parallel-group trial. Clin Drug Investig. 2009; 29(9): 577–90. 10.2165/11317570-000000000-00000 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

