Female mice are protected from space radiation-induced maladaptive responses
- PMID: 30107198
- PMCID: PMC8715721
- DOI: 10.1016/j.bbi.2018.08.008
Female mice are protected from space radiation-induced maladaptive responses
Abstract
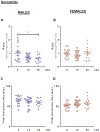
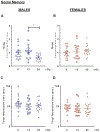
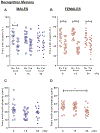
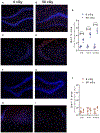
Interplanetary exploration will be humankind's most ambitious expedition and the journey required to do so, is as intimidating as it is intrepid. One major obstacle for successful deep space travel is the possible negative effects of galactic cosmic radiation (GCR) exposure. Here, we investigate for the first time how combined GCR impacts long-term behavioral and cellular responses in male and female mice. We find that a single exposure to simulated GCR induces long-term cognitive and behavioral deficits only in the male cohorts. GCR exposed male animals have diminished social interaction, increased anxiety-like phenotype and impaired recognition memory. Remarkably, we find that the female cohorts did not display any cognitive or behavioral deficits after GCR exposure. Mechanistically, the maladaptive behavioral responses observed only in the male cohorts correspond with microglia activation and synaptic loss in the hippocampus, a brain region involved in the cognitive domains reported here. Furthermore, we measured reductions in AMPA expressing synaptic terminals in the hippocampus. No changes in any of the molecular markers measured here are observed in the females. Taken together these findings suggest that GCR exposure can regulate microglia activity and alter synaptic architecture, which in turn leads to a range of cognitive alterations in a sex dependent manner. These results identify sex-dependent differences in behavioral and cognitive domains revealing promising cellular and molecular intervention targets to reduce GCR-induced chronic cognitive deficits thereby boosting chances of success for humans in deep space missions such as the upcoming Mars voyage.
Keywords: Galactic cosmic ray; Microglia; Radiation; Sex-differences; Synapse.
Copyright © 2018. Published by Elsevier Inc.
Conflict of interest statement
Conflict of interest
The authors report no conflict of interest.
Figures
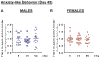



Comment in
-
Perhaps woman are better astronauts?Brain Behav Immun. 2018 Nov;74:47-48. doi: 10.1016/j.bbi.2018.09.021. Epub 2018 Sep 23. Brain Behav Immun. 2018. PMID: 30257190 No abstract available.
References
-
- Cucinotta FA, Nikjoo H, Goodhead DT, 1998. The effects of delta rays on the number of particle-track traversals per cell in laboratory and space exposures. Radiat. Res 150, 115–119. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

