Pan-Filovirus Serum Neutralizing Antibodies in a Subset of Congolese Ebolavirus Infection Survivors
- PMID: 30107445
- PMCID: PMC6217721
- DOI: 10.1093/infdis/jiy453
Pan-Filovirus Serum Neutralizing Antibodies in a Subset of Congolese Ebolavirus Infection Survivors
Abstract
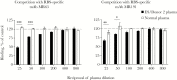
One year after a Zaire ebolavirus (EBOV) outbreak occurred in the Boende Health Zone of the Democratic Republic of the Congo during 2014, we sought to determine the breadth of immune response against diverse filoviruses including EBOV, Bundibugyo (BDBV), Sudan (SUDV), and Marburg (MARV) viruses. After assessing the 15 survivors, 5 individuals demonstrated some degree of reactivity to multiple ebolavirus species and, in some instances, Marburg virus. All 5 of these survivors had immunoreactivity to EBOV glycoprotein (GP) and EBOV VP40, and 4 had reactivity to EBOV nucleoprotein (NP). Three of these survivors showed serologic responses to the 3 species of ebolavirus GPs tested (EBOV, BDBV, SUDV). All 5 samples also exhibited ability to neutralize EBOV using live virus, in a plaque reduction neutralization test. Remarkably, 3 of these EBOV survivors had plasma antibody responses to MARV GP. In pseudovirus neutralization assays, serum antibodies from a subset of these survivors also neutralized EBOV, BDBV, SUDV, and Taï Forest virus as well as MARV. Collectively, these findings suggest that some survivors of naturally acquired ebolavirus infection mount not only a pan-ebolavirus response, but also in less frequent cases, a pan-filovirus neutralizing response.
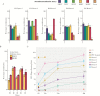
Figures

References
-
- Baseler L, Chertow DS, Johnson KM, Feldmann H, Morens DM. The pathogenesis of Ebola virus disease. Annu Rev Pathol 2017; 12:387–418. - PubMed
-
- Gebre Y, Gebre T, Peters A. The Ebola virus: a review of progress and development in research. Asian Pac J Trop Biomed 2014; 4:928–36.
-
- Khurana S, Fuentes S, Coyle EM, Ravichandran S, Davey RT Jr, Beigel JH. Human antibody repertoire after VSV-Ebola vaccination identifies novel targets and virus-neutralizing IgM antibodies. Nat Med 2016; 22:1439–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

