SYT1-associated neurodevelopmental disorder: a case series
- PMID: 30107533
- PMCID: PMC6113648
- DOI: 10.1093/brain/awy209
SYT1-associated neurodevelopmental disorder: a case series
Abstract
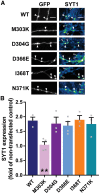
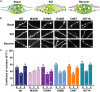
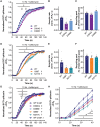
Synaptotagmin 1 (SYT1) is a critical mediator of fast, synchronous, calcium-dependent neurotransmitter release and also modulates synaptic vesicle endocytosis. This paper describes 11 patients with de novo heterozygous missense mutations in SYT1. All mutations alter highly conserved residues, and cluster in two regions of the SYT1 C2B domain at positions Met303 (M303K), Asp304 (D304G), Asp366 (D366E), Ile368 (I368T) and Asn371 (N371K). Phenotypic features include infantile hypotonia, congenital ophthalmic abnormalities, childhood-onset hyperkinetic movement disorders, motor stereotypies, and developmental delay varying in severity from moderate to profound. Behavioural characteristics include sleep disturbance and episodic agitation. Absence of epileptic seizures and normal orbitofrontal head circumference are important negative features. Structural MRI is unremarkable but EEG disturbance is universal, characterized by intermittent low frequency high amplitude oscillations. The functional impact of these five de novo SYT1 mutations has been assessed by expressing rat SYT1 protein containing the equivalent human variants in wild-type mouse primary hippocampal cultures. All mutant forms of SYT1 were expressed at levels approximately equal to endogenous wild-type protein, and correctly localized to nerve terminals at rest, except for SYT1M303K, which was expressed at a lower level and failed to localize at nerve terminals. Following stimulation, SYT1I368T and SYT1N371K relocalized to nerve terminals at least as efficiently as wild-type SYT1. However, SYT1D304G and SYT1D366E failed to relocalize to nerve terminals following stimulation, indicative of impairments in endocytic retrieval and trafficking of SYT1. In addition, the presence of SYT1 variants at nerve terminals induced a slowing of exocytic rate following sustained action potential stimulation. The extent of disturbance to synaptic vesicle kinetics is mirrored by the severity of the affected individuals' phenotypes, suggesting that the efficiency of SYT1-mediated neurotransmitter release is critical to cognitive development. In summary, de novo dominant SYT1 missense mutations are associated with a recognizable neurodevelopmental syndrome, and further cases can now be diagnosed based on clinical features, electrophysiological signature and mutation characteristics. Variation in phenotype severity may reflect mutation-specific impact on the diverse physiological functions of SYT1.
Figures


References
-
- Ebrahimi-Fakhari D, Saffari A, Westenberger A, Klein C. The evolving spectrum of PRRT2-associated paroxysmal diseases. Brain 2015; 138 (Pt 12): 3476–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

