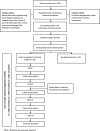
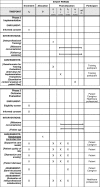
The Heidelberg Milestones Communication Approach (MCA) for patients with prognosis <12 months: protocol for a mixed-methods study including a randomized controlled trial
- PMID: 30107809
- PMCID: PMC6092809
- DOI: 10.1186/s13063-018-2814-1
The Heidelberg Milestones Communication Approach (MCA) for patients with prognosis <12 months: protocol for a mixed-methods study including a randomized controlled trial
Abstract
Background: The care needs of patients with a limited prognosis (<12 months median) are complex and dynamic. Patients and caregivers must cope with many challenges, including physical symptoms and disabilities, uncertainty. and compromised self-efficacy. Healthcare is often characterized by disruptions in the transition between healthcare providers. The Milestones Communication Approach (MCA) is a structured, proactive, interprofessional concept that involves physicians and nurses and is aimed at providing coherent care across the disease trajectory. This study aims to evaluate these aspects of MCA: (1) the training of healthcare professionals, (2) implementation context and outcomes, (3) patient outcomes, and (4) effects on interprofessional collaboration.
Methods/design: A multiphase mixed-methods design will be used for the study. A total of 100 patients and 120 healthcare professionals in a specialized oncology hospital will be involved. The training outcomes will be documented using a questionnaire. Implementation context and outcomes will be explored through semi-structured interviews and written questionnaires with healthcare professionals and with the training participants and through a content analysis of patient files. Patient outcomes will be assessed in a pragmatic non-blinded randomized controlled trial and in qualitative interviews with patients and caregivers. Trial outcomes are supportive care needs (SCNS-SF34-G), quality of life (SeiQol and Fact-L), depression and anxiety symptoms (PHQ-4), and distress (Distress Thermometer). Qualitative semi-structured interviews on patients' views will focus on shared decision-making, communication needs, feeling empathy, and further utilization of healthcare services. Interprofessional collaboration will be explored using the UWE-IP-D before the implementation of MCA (t0) and after 3 (t1), 9 (t2), and 12 (t3) months.
Discussion: Using guideline-concordant early palliative care, MCA aims to foster patient-centered communication with shared decision-making and facilitation of advance care planning including end-of-life decisions, thus increasing patient quality of life and decreasing aggressive medical care at the end of life. It is assumed that the communication skills training and interprofessional coaching will improve the communication behavior of healthcare providers and influence team communications and team processes.
Trial registration: German Clinical Trials Register, DRKS00013649 and DRKS00013469. Registered on 22 December 2017.
Keywords: Communication; Complex intervention; Implementation; Interprofessional relations; Multiphase mixed method; Palliative care; Prognosis; Prognostic awareness; Randomized controlled trial.
Conflict of interest statement
Ethics approval and consent to participate
See attachment “Confirmation Approval” of the Ethics Committee of the University Hospital Heidelberg, Germany (Additional file 3).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Villalobos M, Coulibaly K, Hermann K, Kamradt M, Wensing M, Siegle A, Kuon J, Eschbach C, Tessmer G, Winkler E, et al. A longitudinal communication approach in advanced lung cancer: a qualitative study of patients’, relatives’ and staff’s perspectives. Eur J Cancer Care (Engl). 2018;27(2):e12794. 10.1111/ecc.12794. Epub 2017 Nov 23. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases