The efficacy and adverse events of mTOR inhibitors in lymphangioleiomyomatosis: systematic review and meta-analysis
- PMID: 30107845
- PMCID: PMC6092843
- DOI: 10.1186/s13023-018-0874-7
The efficacy and adverse events of mTOR inhibitors in lymphangioleiomyomatosis: systematic review and meta-analysis
Abstract
Background: Lymphangioleiomyomatosis (LAM) is a rare lung disease and the mammalian target of the rapamycin (mTOR) inhibitors has been used as an effective therapy. Here we conducted a systematic review and meta-analysis with the aims to quantify the efficacy and safety of mTOR inhibitors in LAM patients.
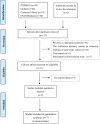
Methods: The following databases were searched for clinical trials regarding LAM patients treated with mTOR inhibitors until December 2017: Pubmed, Embase, Cochrane Library and OVID medicine. Random effect models were used for the quantitative analysis.
Results: Nine eligible studies were included in our systematic review, 7 of which were used for the meta-analysis. In LAM patients, mTOR inhibitors improved forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) significantly, with the weighted mean difference (WMD) 0.15 L (95%CI: 0.08 to 0.22, P < 0.01, I2 = 0%) and 0.22 L (95%: 0.11 to 0.32, P < 0.01, I2 = 0%) respectively. There was no significant change in neither the diffusing capacity for carbon monoxide (WMD: 0.51 ml/mm Hg/min, 95%CI: -0.48 to 1.49, P = 0.31, I2 = 0%) nor 6-min walking distance (WMD: 5.29 m, 95%CI: -18.01 to 28.59, P = 0.66, I2 = 1%). The weighted partial response rate was 0.68 (95%CI: 0.53 to 0.84, P < 0.01, I2 = 72%) for renal angiomylipoma. The cumulative incidence rates of common safety events were 50, 40, 23, 20 and 19% for oral mucositis, hyperlipidemia, headache, bone marrow suppression, and diarrhea, respectively. And most events were low grade and tolerant.
Conclusions: In LAM patients, there are improvements of FEV1 and FVC after the application of mTOR inhibitors and over a half achieved the shrinkage of renal angiomyolipoma.
Trial registration: PROSPERO registration number: CRD42018085470. Registered 22 January 2018.
Keywords: Lymphangioleiomyomatosis; Meta-analysis; mTOR inhibitors.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

