A Drosophila model of combined D-2- and L-2-hydroxyglutaric aciduria reveals a mechanism linking mitochondrial citrate export with oncometabolite accumulation
- PMID: 30108060
- PMCID: PMC6177012
- DOI: 10.1242/dmm.035337
A Drosophila model of combined D-2- and L-2-hydroxyglutaric aciduria reveals a mechanism linking mitochondrial citrate export with oncometabolite accumulation
Abstract
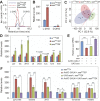
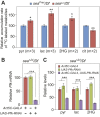
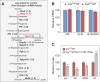
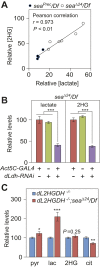
The enantiomers of 2-hydroxyglutarate (2HG) are potent regulators of metabolism, chromatin modifications and cell fate decisions. Although these compounds are associated with tumor metabolism and commonly referred to as oncometabolites, both D- and L-2HG are also synthesized by healthy cells and likely serve endogenous functions. The metabolic mechanisms that control 2HG metabolism in vivo are poorly understood. One clue towards how cells regulate 2HG levels has emerged from an inborn error of metabolism known as combined D- and L-2HG aciduria (D-/L-2HGA), which results in elevated D- and L-2HG accumulation. Because this disorder is caused by mutations in the mitochondrial citrate transporter (CIC), citrate must somehow govern 2HG metabolism in healthy cells. The mechanism linking citrate and 2HG, however, remains unknown. Here, we use the fruit fly Drosophila melanogaster to elucidate a metabolic link between citrate transport and L-2HG accumulation. Our study reveals that the Drosophila gene scheggia (sea), which encodes the fly CIC homolog, dampens glycolytic flux and restricts L-2HG accumulation. Moreover, we find that sea mutants accumulate excess L-2HG owing to elevated lactate production, which inhibits L-2HG degradation by interfering with L-2HG dehydrogenase activity. This unexpected result demonstrates that citrate indirectly regulates L-2HG stability and reveals a feedback mechanism that coordinates L-2HG metabolism with glycolysis and the tricarboxylic acid cycle. Finally, our study also suggests a potential strategy for preventing L-2HG accumulation in human patients with CIC deficiency.This article has an associated First Person interview with the first author of the paper.
Keywords: 2-Hydroxyglutarate; L2HGDH; Oncometabolite; SLC25A1; Scheggia.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

References
-
- Backhaus B., Sulkowski E. and Schlote F. (1984). A semi-synthetic, general-purpose medium for Drosophila melanogaster. Dros. Inf. Serv 60, 210-212.
-
- Becker-Kettern J., Paczia N., Conrotte J. F., Kay D. P., Guignard C., Jung P. P. and Linster C. L. (2016). Saccharomyces cerevisiae forms D-2-hydroxyglutarate and couples its degradation to D-lactate formation via a cytosolic transhydrogenase. J. Biol. Chem. 291, 6036-6058. 10.1074/jbc.M115.704494 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

