TFEB enhances astroglial uptake of extracellular tau species and reduces tau spreading
- PMID: 30108137
- PMCID: PMC6122971
- DOI: 10.1084/jem.20172158
TFEB enhances astroglial uptake of extracellular tau species and reduces tau spreading
Abstract
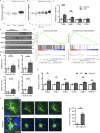
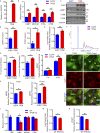
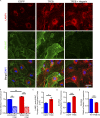
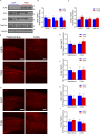
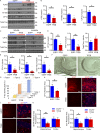
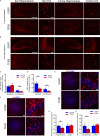
The progression of tau pathology in Alzheimer's disease follows a stereotyped pattern, and recent evidence suggests a role of synaptic connections in this process. Astrocytes are well positioned at the neuronal synapse to capture and degrade extracellular tau as it transits the synapse and hence could potentially have the ability to inhibit tau spreading and delay disease progression. Our study shows increased expression and activity of Transcription Factor EB (TFEB), a master regulator of lysosomal biogenesis, in response to tau pathology in both human brains with dementia and transgenic mouse models. Exogenous TFEB expression in primary astrocytes enhances tau fibril uptake and lysosomal activity, while TFEB knockout has the reverse effect. In vivo, induced TFEB expression in astrocytes reduces pathology in the hippocampus of PS19 tauopathy mice, as well as prominently attenuates tau spreading from the ipsilateral to the contralateral hippocampus in a mouse model of tau spreading. Our study suggests that astrocytic TFEB plays a functional role in modulating extracellular tau and the propagation of neuronal tau pathology in tauopathies such as Alzheimer's disease.
© 2018 Martini-Stoica et al.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

