Metabolism of a synthetic compared with a natural therapeutic pulmonary surfactant in adult mice
- PMID: 30108154
- PMCID: PMC6168297
- DOI: 10.1194/jlr.M085431
Metabolism of a synthetic compared with a natural therapeutic pulmonary surfactant in adult mice
Abstract
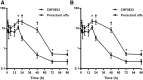
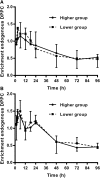
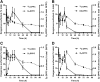
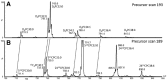
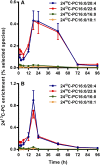
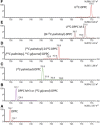
Secreted pulmonary surfactant phosphatidylcholine (PC) has a complex intra-alveolar metabolism that involves uptake and recycling by alveolar type II epithelial cells, catabolism by alveolar macrophages, and loss up the bronchial tree. We compared the in vivo metabolism of animal-derived poractant alfa (Curosurf) and a synthetic surfactant (CHF5633) in adult male C57BL/6 mice. The mice were dosed intranasally with either surfactant (80 mg/kg body weight) containing universally 13C-labeled dipalmitoyl PC (DPPC) as a tracer. The loss of [U13C]DPPC from bronchoalveolar lavage and lung parenchyma, together with the incorporation of 13C-hydrolysis fragments into new PC molecular species, was monitored by electrospray ionization tandem mass spectrometry. The catabolism of CHF5633 was considerably delayed compared with poractant alfa, the hydrolysis products of which were cleared more rapidly. There was no selective resynthesis of DPPC and, strikingly, acyl remodeling resulted in preferential synthesis of polyunsaturated PC species. In conclusion, both surfactants were metabolized by similar pathways, but the slower catabolism of CHF5633 resulted in longer residence time in the airways and enhanced recycling of its hydrolysis products into new PC species.
Keywords: acyl remodeling; mass spectrometry; molecular species; phosphatidylcholine synthesis; stable isotopes.
Copyright © 2018 Madsen et al. Published by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Avery M. E., and Mead J.. 1959. Surface properties in relation to atelectasis and hyaline membrane disease. AMA J. Dis. Child. 97: 517–523. - PubMed
-
- Robertson B. 1998. Surfactant inactivation and surfactant therapy in acute respiratory distress syndrome (ARDS). Monaldi Arch. Chest Dis. 53: 64–69. - PubMed
-
- Lopez-Rodriguez E., and Perez-Gil J.. 2014. Structure-function relationships in pulmonary surfactant membranes: from biophysics to therapy. Biochim. Biophys. Acta. 1838: 1568–1585. - PubMed
-
- Echaide M., Autilio C., Arroyo R., and Perez-Gil J.. 2017. Restoring pulmonary surfactant membranes and films at the respiratory surface. Biochim. Biophys. Acta. 1859: 1725–1739. - PubMed
-
- Arbibe L., Koumanov K., Vial D., Rougeot C., Faure G., Havet N., Longacre S., Vargaftig B. B., Bereziat G., Voelker D. R., et al. . 1998. Generation of lyso-phospholipids from surfactant in acute lung injury is mediated by type-II phospholipase A2 and inhibited by a direct surfactant protein A-phospholipase A2 protein interaction. J. Clin. Invest. 102: 1152–1160. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

