Molecular predictors of prevention of recurrence in HCC with sorafenib as adjuvant treatment and prognostic factors in the phase 3 STORM trial
- PMID: 30108162
- PMCID: PMC6580745
- DOI: 10.1136/gutjnl-2018-316408
Molecular predictors of prevention of recurrence in HCC with sorafenib as adjuvant treatment and prognostic factors in the phase 3 STORM trial
Abstract
Objective: Sorafenib is the standard systemic therapy for advanced hepatocellular carcinoma (HCC). Survival benefits of resection/local ablation for early HCC are compromised by 70% 5-year recurrence rates. The phase 3 STORM trial comparing sorafenib with placebo as adjuvant treatment did not achieve its primary endpoint of improving recurrence-free survival (RFS). The biomarker companion study BIOSTORM aims to define (A) predictors of recurrence prevention with sorafenib and (B) prognostic factors with B level of evidence.
Design: Tumour tissue from 188 patients randomised to receive sorafenib (83) or placebo (105) in the STORM trial was collected. Analyses included gene expression profiling, targeted exome sequencing (19 known oncodrivers), immunohistochemistry (pERK, pVEGFR2, Ki67), fluorescence in situ hybridisation (VEGFA) and immunome. A gene signature capturing improved RFS in sorafenib-treated patients was generated. All 70 RFS events were recurrences, thus time to recurrence equalled RFS. Predictive and prognostic value was assessed using Cox regression models and interaction test.
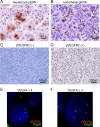
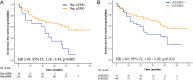
Results: BIOSTORM recapitulates clinicopathological characteristics of STORM. None of the biomarkers tested (related to angiogenesis and proliferation) or previously proposed gene signatures, or mutations predicted sorafenib benefit or recurrence. A newly generated 146-gene signature identifying 30% of patients captured benefit to sorafenib in terms of RFS (p of interaction=0.04). These sorafenib RFS responders were significantly enriched in CD4+ T, B and cytolytic natural killer cells, and lacked activated adaptive immune components. Hepatocytic pERK (HR=2.41; p=0.012) and microvascular invasion (HR=2.09; p=0.017) were independent prognostic factors.
Conclusion: In BIOSTORM, only hepatocytic pERK and microvascular invasion predicted poor RFS. No mutation, gene amplification or previously proposed gene signatures predicted sorafenib benefit. A newly generated multigene signature associated with improved RFS on sorafenib warrants further validation.
Trial registration number: NCT00692770.
Keywords: cancer; clinical trials; hepatocellular carcinoma; molecular oncology; tumour markers.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JML, JB, VM and AEC received research support and consultancy fees from Bayer. AV and SS received consultancy fees from Bayer. CP and GM are employees of Bayer HealthCare Pharmaceuticals.
Figures


References
-
- European Association for the study of the liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. European Association for the Study of the Liver. J Hepatol 2018;69:182–236. - PubMed
-
- Kudo M. A randomised phase 3 trial of lenvatinib versus sorafenib in firstline treatment of patients with unresectable hepatocellular carcinoma. Lancet 2018;391:1163–73. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
