Highly efficient cellular uptake of a cell-penetrating peptide (CPP) derived from the capsid protein of porcine circovirus type 2
- PMID: 30108178
- PMCID: PMC6166719
- DOI: 10.1074/jbc.RA118.004823
Highly efficient cellular uptake of a cell-penetrating peptide (CPP) derived from the capsid protein of porcine circovirus type 2
Abstract
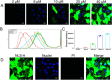
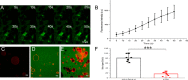
Porcine circovirus type 2 (PCV2) is one of the smallest, nonenveloped, single-stranded DNA viruses. The PCV2 capsid protein (Cap) is the sole viral structural protein and main antigenic determinant. Previous sequence analysis has revealed that the N terminus of the PCV2 Cap contains a nuclear localization signal (NLS) enriched in positively charged residues. Here, we report that PCV2's NLS can function as a cell-penetrating peptide (CPP). We observed that this NLS can carry macromolecules, e.g. enhanced GFP (EGFP), into cells when they are fused to the NLS, indicating that it can function as a CPP, similar to the classical CPP derived from HIV type 1 transactivator of transcription protein (HIV TAT). We also found that the first 17 residues of the NLS (NLS-A) have a key role in cellular uptake. In addition to entering cells via multiple endocytic processes, NLS-A was also rapidly internalized via direct translocation enabled by increased membrane permeability and was evenly distributed throughout cells when its concentration in cell cultures was ≥10 μm Of note, cellular NLS-A uptake was ∼10 times more efficient than that of HIV TAT. We inferred that the externalized NLS of the PCV2 Cap may accumulate to a high concentration (≥10 μm) at a local membrane area, increasing membrane permeability to facilitate viral entry into the cell to release its genome into a viral DNA reproduction center. We conclude that NLS-A has potential as a versatile vehicle for shuttling foreign molecules into cells, including pharmaceuticals for therapeutic interventions.
Keywords: Circoviridae; capsid protein; cell-penetrating peptide (CPP); cellular uptake; endocytosis; intracellular trafficking; membrane; membrane permeability; nuclear localization signal (NLS); permeability; porcine circovirus virus (PCV); protein delivery; transport vector.
© 2018 Yu et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

References
-
- Tischer I., Rasch R., and Tochtermann G. (1974) Characterization of papovavirus-and picornavirus-like particles in permanent pig kidney cell lines. Zentralbl. Bakteriol. Orig. A 226, 153–167 - PubMed
-
- Palinski R., Piñeyro P., Shang P., Yuan F., Guo R., Fang Y., Byers E., and Hause B. M. (2017) A novel porcine circovirus distantly related to known circoviruses is associated with porcine dermatitis and nephropathy syndrome and reproductive failure. J. Virol. 91, e01879–16 10.1128/JVI.01879-16 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

