Revealing circadian mechanisms of integration and resilience by visualizing clock proteins working in real time
- PMID: 30108211
- PMCID: PMC6092398
- DOI: 10.1038/s41467-018-05438-4
Revealing circadian mechanisms of integration and resilience by visualizing clock proteins working in real time
Abstract
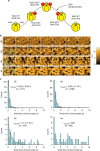
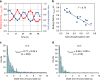
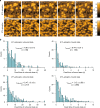
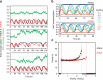
The circadian clock proteins KaiA, KaiB, and KaiC reconstitute a remarkable circa-24 h oscillation of KaiC phosphorylation that persists for many days in vitro. Here we use high-speed atomic force microscopy (HS-AFM) to visualize in real time and quantify the dynamic interactions of KaiA with KaiC on sub-second timescales. KaiA transiently interacts with KaiC, thereby stimulating KaiC autokinase activity. As KaiC becomes progressively more phosphorylated, KaiA's affinity for KaiC weakens, revealing a feedback of KaiC phosphostatus back onto the KaiA-binding events. These non-equilibrium interactions integrate high-frequency binding and unbinding events, thereby refining the period of the longer term oscillations. Moreover, this differential affinity phenomenon broadens the range of Kai protein stoichiometries that allow rhythmicity, explaining how the oscillation is resilient in an in vivo milieu that includes noise. Therefore, robustness of rhythmicity on a 24-h scale is explainable by molecular events occurring on a scale of sub-seconds.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Intermolecular associations determine the dynamics of the circadian KaiABC oscillator.Proc Natl Acad Sci U S A. 2010 Aug 17;107(33):14805-10. doi: 10.1073/pnas.1002119107. Epub 2010 Aug 2. Proc Natl Acad Sci U S A. 2010. PMID: 20679240 Free PMC article.
-
Damped circadian oscillation in the absence of KaiA in Synechococcus.Nat Commun. 2020 May 7;11(1):2242. doi: 10.1038/s41467-020-16087-x. Nat Commun. 2020. PMID: 32382052 Free PMC article.
-
Analysis of KaiA-KaiC protein interactions in the cyano-bacterial circadian clock using hybrid structural methods.EMBO J. 2006 May 3;25(9):2017-28. doi: 10.1038/sj.emboj.7601086. Epub 2006 Apr 20. EMBO J. 2006. PMID: 16628225 Free PMC article.
-
A cyanobacterial circadian clock based on the Kai oscillator.Cold Spring Harb Symp Quant Biol. 2007;72:47-55. doi: 10.1101/sqb.2007.72.029. Cold Spring Harb Symp Quant Biol. 2007. PMID: 18419262 Review.
-
Diversity of KaiC-based timing systems in marine Cyanobacteria.Mar Genomics. 2014 Apr;14:3-16. doi: 10.1016/j.margen.2013.12.006. Epub 2014 Jan 3. Mar Genomics. 2014. PMID: 24388874 Review.
Cited by
-
Bayesian modeling reveals metabolite-dependent ultrasensitivity in the cyanobacterial circadian clock.Mol Syst Biol. 2020 Jun;16(6):e9355. doi: 10.15252/msb.20199355. Mol Syst Biol. 2020. PMID: 32496641 Free PMC article.
-
The priming phosphorylation of KaiC is activated by the release of its autokinase autoinhibition.PNAS Nexus. 2025 Apr 28;4(5):pgaf136. doi: 10.1093/pnasnexus/pgaf136. eCollection 2025 May. PNAS Nexus. 2025. PMID: 40352643 Free PMC article.
-
Regulation mechanisms of the dual ATPase in KaiC.Proc Natl Acad Sci U S A. 2022 May 10;119(19):e2119627119. doi: 10.1073/pnas.2119627119. Epub 2022 May 4. Proc Natl Acad Sci U S A. 2022. PMID: 35507871 Free PMC article.
-
The ESCRT-III isoforms CHMP2A and CHMP2B display different effects on membranes upon polymerization.BMC Biol. 2021 Apr 8;19(1):66. doi: 10.1186/s12915-021-00983-9. BMC Biol. 2021. PMID: 33832485 Free PMC article.
-
Role of the reaction-structure coupling in temperature compensation of the KaiABC circadian rhythm.PLoS Comput Biol. 2022 Sep 6;18(9):e1010494. doi: 10.1371/journal.pcbi.1010494. eCollection 2022 Sep. PLoS Comput Biol. 2022. PMID: 36067222 Free PMC article.
References
-
- Dunlap, J. C., DeCoursey, P. J. & Loros, J. J. Chronobiology: Biological Timekeeping. (Sinauer Associates, Sunderland, MA, 2003).
Publication types
MeSH terms
Substances
Grants and funding
- 17H06121/Japan Society for the Promotion of Science (JSPS)/International
- JP24227005/Japan Society for the Promotion of Science (JSPS)/International
- JP16H00830/Japan Society for the Promotion of Science (JSPS)/International
- JPMJCR13M1/Japan Science and Technology Agency (JST)/International
- U54 HD083211/HD/NICHD NIH HHS/United States
- JP15H03540/Japan Society for the Promotion of Science (JSPS)/International
- JP26119003/Japan Society for the Promotion of Science (JSPS)/International
- R37 GM067152/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)/International
- R37 GM067152/GM/NIGMS NIH HHS/United States
- JP16H00758/Japan Society for the Promotion of Science (JSPS)/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

