Strong increase in the autofluorescence of cells signals struggle for survival
- PMID: 30108248
- PMCID: PMC6092379
- DOI: 10.1038/s41598-018-30623-2
Strong increase in the autofluorescence of cells signals struggle for survival
Abstract
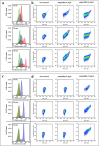
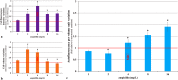
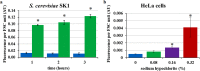
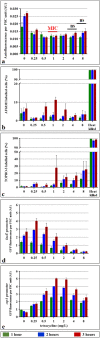
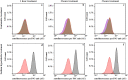
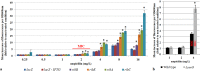
Prokaryotic and eukaryotic cells exhibit an intrinsic natural fluorescence due to the presence of fluorescent cellular structural components and metabolites. Therefore, cellular autofluorescence (AF) is expected to vary with the metabolic states of cells. We examined how exposure to the different stressors changes the AF of Escherichia coli cells. We observed that bactericidal treatments increased green cellular AF, and that de novo protein synthesis was required for the observed AF increase. Excitation and emission spectra and increased expression of the genes from the flavin biosynthesis pathway, strongly suggested that flavins are major contributors to the increased AF. An increased expression of genes encoding diverse flavoproteins which are involved in energy production and ROS detoxification, indicates a cellular strategy to cope with severe stresses. An observed increase in AF under stress is an evolutionary conserved phenomenon as it occurs not only in cells from different bacterial species, but also in yeast and human cells.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

