Isoform-Specific Compensation of Cyclooxygenase (Ptgs) Genes during Implantation and Late-Stage Pregnancy
- PMID: 30108257
- PMCID: PMC6092371
- DOI: 10.1038/s41598-018-30636-x
Isoform-Specific Compensation of Cyclooxygenase (Ptgs) Genes during Implantation and Late-Stage Pregnancy
Abstract
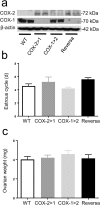
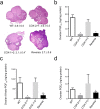
The participation of cyclooxygenase (COX) in embryo implantation and parturition has been studied extensively. However, the distinct role of the two COX isoforms in these processes still remains unclear. Using three characterized mouse lines where the Ptgs1 and Ptgs2 genes substitute for one another, this study focused on the reproductive significance of their distinct roles and potential biological substitution. In both non-gravid and gravid uteri, the knock-in COX-2 is expressed constitutively, whereas the knock-in COX-1 is slightly induced in early implantation. The delayed onset of parturition previously found in COX-1 null mice was corrected by COX-2 exchange in COX-2>COX-1 mice, with normal term pregnancy, gestation length and litter size. In contrast, loss of native COX-2 in COX-1>COX-2 mice resulted in severely impaired reproductive functions. Knock-in COX-1 failed to substitute for the loss of COX-2 in COX-1>COX-2 mice during implantation, indicating that COX-1 may be replaced by COX-2, but not vice versa. A panel of prostaglandins detected in uterus and ovary demonstrates that prostaglandin biosynthesis preferentially depends on native COX-1, but not COX-2. More interestingly, preferential compensations by the COX isoforms were sustained despite weak dependency on their role in prostaglandin biosynthesis in the uterus and ovary.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Restoration of on-time embryo implantation corrects the timing of parturition in cytosolic phospholipase A2 group IVA deficient mice.Biol Reprod. 2009 Dec;81(6):1131-8. doi: 10.1095/biolreprod.109.079061. Epub 2009 Aug 14. Biol Reprod. 2009. PMID: 19684335 Free PMC article.
-
Differential compensation of two cyclooxygenases in renal homeostasis is independent of prostaglandin-synthetic capacity under basal conditions.FASEB J. 2018 Oct;32(10):5326-5337. doi: 10.1096/fj.201800252R. Epub 2018 Apr 20. FASEB J. 2018. PMID: 29676940 Free PMC article.
-
Dysregulation of EGF family of growth factors and COX-2 in the uterus during the preattachment and attachment reactions of the blastocyst with the luminal epithelium correlates with implantation failure in LIF-deficient mice.Mol Endocrinol. 2000 Aug;14(8):1147-61. doi: 10.1210/mend.14.8.0498. Mol Endocrinol. 2000. PMID: 10935540
-
Prostaglandins: critical roles in pregnancy and colon cancer.Curr Biol. 1998 Jan 29;8(3):R87-9. doi: 10.1016/s0960-9822(98)70053-3. Curr Biol. 1998. PMID: 9443907 Review.
-
The role of cyclooxygenases in inflammation, cancer, and development.Oncogene. 1999 Dec 20;18(55):7908-16. doi: 10.1038/sj.onc.1203286. Oncogene. 1999. PMID: 10630643 Review.
Cited by
-
Effect of NETs/COX-2 pathway on immune microenvironment and metastasis in gastric cancer.Front Immunol. 2023 Apr 20;14:1177604. doi: 10.3389/fimmu.2023.1177604. eCollection 2023. Front Immunol. 2023. PMID: 37153547 Free PMC article.
-
The Molecular Mechanisms of HLA-G Regulatory Function on Immune Cells during Early Pregnancy.Biomolecules. 2023 Aug 3;13(8):1213. doi: 10.3390/biom13081213. Biomolecules. 2023. PMID: 37627278 Free PMC article. Review.
-
Roles of lipid mediators in early pregnancy events.Reprod Med Biol. 2024 Jul 15;23(1):e12597. doi: 10.1002/rmb2.12597. eCollection 2024 Jan-Dec. Reprod Med Biol. 2024. PMID: 39010880 Free PMC article. Review.
-
Signaling Pathways Mediating Bradykinin-Induced Contraction in Murine and Human Detrusor Muscle.Front Med (Lausanne). 2022 Jan 20;8:745638. doi: 10.3389/fmed.2021.745638. eCollection 2021. Front Med (Lausanne). 2022. PMID: 35127739 Free PMC article.
-
Spatiotemporally distinct roles of cyclooxygenase-1 and cyclooxygenase-2 at fetomaternal interface in mice.JCI Insight. 2024 Aug 27;9(19):e181865. doi: 10.1172/jci.insight.181865. JCI Insight. 2024. PMID: 39377223 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

