Panic results in unique molecular and network changes in the amygdala that facilitate fear responses
- PMID: 30108314
- PMCID: PMC6410355
- DOI: 10.1038/s41380-018-0119-0
Panic results in unique molecular and network changes in the amygdala that facilitate fear responses
Abstract
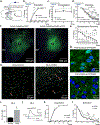
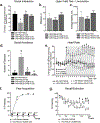
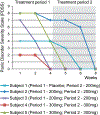
Recurrent panic attacks (PAs) are a common feature of panic disorder (PD) and post-traumatic stress disorder (PTSD). Several distinct brain regions are involved in the regulation of panic responses, such as perifornical hypothalamus (PeF), periaqueductal gray, amygdala and frontal cortex. We have previously shown that inhibition of GABA synthesis in the PeF produces panic-vulnerable rats. Here, we investigate the mechanisms by which a panic-vulnerable state could lead to persistent fear. We first show that optogenetic activation of glutamatergic terminals from the PeF to the basolateral amygdala (BLA) enhanced the acquisition, delayed the extinction and induced the persistence of fear responses 3 weeks later, confirming a functional PeF-amygdala pathway involved in fear learning. Similar to optogenetic activation of PeF, panic-prone rats also exhibited delayed extinction. Next, we demonstrate that panic-prone rats had altered inhibitory and enhanced excitatory synaptic transmission of the principal neurons, and reduced protein levels of metabotropic glutamate type 2 receptor (mGluR2) in the BLA. Application of an mGluR2-positive allosteric modulator (PAM) reduced glutamate neurotransmission in the BLA slices from panic-prone rats. Treating panic-prone rats with mGluR2 PAM blocked sodium lactate (NaLac)-induced panic responses and normalized fear extinction deficits. Finally, in a subset of patients with comorbid PD, treatment with mGluR2 PAM resulted in complete remission of panic symptoms. These data demonstrate that a panic-prone state leads to specific reduction in mGluR2 function within the amygdala network and facilitates fear, and mGluR2 PAMs could be a targeted treatment for panic symptoms in PD and PTSD patients.
Conflict of interest statement
CONFLICT OF INTERESTS
Drs. L Ver Donck, M Ceusters and JM Kent are employees of Janssen. This work was supported by research grants to Indiana University with (Drs. Shekhar, Molosh and Johnson as PIs) from Janssen, but these authors have no other commercial conflicts.
Figures


References
-
- Stein DJ, Bouwer C. A neuro-evolutionary approach to the anxiety disorders. JAnxietyDisord 1997; 11(4): 409–429. - PubMed
-
- DSM- V. Diagnostic and Statistical Manual - Fifth Edn. (DSM - V) American Psychiatric Association: Washington, DC, 2013.
-
- Rasche D, Foethke D, Gliemroth J, Tronnier VM. [Deep brain stimulation in the posterior hypothalamus for chronic cluster headache. Case report and review of the literature]. Schmerz 2006; 20(5): 439–444. - PubMed
-
- Wilent WB, Oh MY, Buetefisch C, Bailes JE, Cantella D, Angle C et al. Mapping of microstimulation evoked responses and unit activity patterns in the lateral hypothalamic area recorded in awake humans. Technical note. Journal of neurosurgery 2011; 115(2): 295–300. - PubMed
-
- Wilent WB, Oh MY, Buetefisch CM, Bailes JE, Cantella D, Angle C et al. Induction of panic attack by stimulation of the ventromedial hypothalamus. Journal of neurosurgery 2010; 112(6): 1295–1298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

